 |
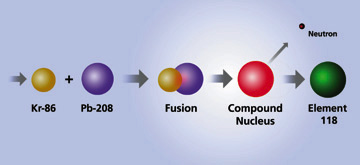
| A low-energy collision of Krypton and Lead ions creates Element 118 |
|
 |
Editor's note: On July 27, 2001, the
results
reported below were retracted through a correspondence
with Physical Review Letters.
They existed for less than an instant but were
the subjects of front page stories in the San Francisco
Chronicle and Examiner and the San Jose Mercury News,
as well as featured on evening news broadcasts. Media
from far afield, including the Times of New York, London,
and Los Angeles also joined in to announce the discovery
of two new "superheavy" elements at Berkeley Lab.
Element 118 and its immediate decay product, element 116,
were discovered at the 88-Inch Cyclotron by bombarding targets
of lead with an intense beam of high-energy krypton ions.
Although both new elements almost instantly decayed into
other elements, the sequence of decay events was consistent
with theories that have long predicted an "island of stability"
for nuclei with approximately 114 protons and 184 neutrons.
Superheavy elements within this island are predicted to
have half-lives that would be measured in terms of years
or even hundreds of years.
"We jumped over a sea of instability onto an island of stability
that theories have been predicting since the 1970s," said
nuclear physicist Victor Ninov, the first author of a paper
that has been submitted to Physical Review Letters.
Said Ken Gregorich, a nuclear chemist who led the discovery
team, "We were able to produce these superheavies using
a reaction that, until a few months ago, we had not considered
using. However, theoretician Robert Smolanczuk, a visiting
Fulbright scholar from the Soltan Institute for Nuclear
Studies in Poland, calculated that this reaction should
have particularly favorable production rates.
"Our unexpected success in producing these superheavy elements
opens up a whole world of possibilities using similar reactions:
new elements and isotopes, tests of nuclear stability and
mass models, and a new understanding of nuclear reactions
for the production of heavy elements."
Gregorich and Ninov are members of Berkeley Lab's Nuclear
Science Division (NSD). Other NSD participants included
Albert Ghiorso and Darleane Hoffman, long-time leaders in
the search for superheavy elements, plus Diana Lee, Heino
Nitsche, Wladyslaw Swiatecki, Uwe Kirbach, and Carola Laue.
Walter Loveland, on sabbatical from Oregon State University,
also made major contributions to this work. Contributions
were also made by UC Berkeley graduate students Jeb Adams,
Joshua Patin, Dawn Shaughnessy, Dan Strellis, and Philip
Wilk.
The isotope of element 118 with mass number 293 identified
at Berkeley Lab contains 118 protons and 175 neutrons in
its nucleus. By comparison, the heaviest element found in
nature in sizeable quantities is uranium, which in its most
common form contains 92 protons and 146 neutrons. Transuranic
elements in the periodic table can only be synthesized in
nuclear reactors or particle accelerators. Though often
short-lived, these artificial elements provide scientists
with valuable insights into the structure of atomic nuclei
and offer opportunities to study the chemical properties
of the heaviest elements beyond uranium.
Within less than a millisecond after its creation, the element
118 nucleus decays by emitting an alpha particle (a helium
nucleus consisting of two protons and two neutrons), leaving
behind an isotope of element 116 with mass number 289, containing
116 protons and 173 neutrons. This daughter element 116
is also radioactive, alpha-decaying to an isotope of element
114. The chain of successive alpha decays continues down
the even numbered elements until at least element 106.
"In these experiments, observation of a chain of six high-energy
alpha decays within about one second unambiguously signaled
the production and decay of element 118," says Gregorich.
"During 11 days of experiments, three such alpha-decay chains
were observed, indicating production of three atoms of element
118. The decay energies and lifetimes measured for these
new isotopes of elements 118, 116, 114, 112, 110, 108, and
106 provide strong support for the existence of the predicted
island of stability."
Referring to these results, discovery team member Hoffman
said, "After a 30-year search, this discovery is extremely
gratifying. I only wish Glenn Seaborg had been alive to
see these results." Seaborg, the recently deceased Nobel
laureate chemist and co-discoverer of plutonium and nine
other transuranic elements, was one of the earliest and
most outspoken advocates of experiments to reach the predicted
island of stability.
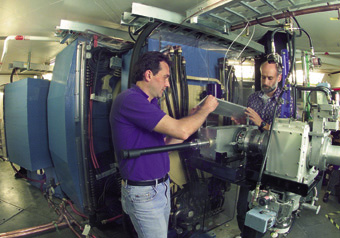 |
| Victor Ninov and Ken Gregorich of Nuclear Science at the new Berkeley Gas-Filled Separator (BGS). Researchers cited the innovative design of the BGS as the key to the success of the experiment. |
|
 |
Elements 118 and 116 were discovered by accelerating a beam of krypton-86 ions to an energy of 449 million electron volts and directing the beam into targets of lead-208. This bombardment resulted in a fusion reaction that yielded heavy compound nuclei at low excitation energies.
During the last several years, low excitation energy fusion reactions failed to take scientists beyond element 112. It was assumed that production rates for heavier elements were too small to extend the periodic table further using this approach. However, Smolanczuk's recent calculations indicating increased production rates for the Kr-86 + Pb-208 fusion reaction prompted the experimental search for element 118 at Berkeley Lab.
The key to the success of this experiment was the newly constructed Berkeley Gas-filled Separator (BGS), the design of which was based on a proposal by discovery team member Ghiorso. Said Gregorich, "The innovative design of the BGS has resulted in a separator with unsurpassed efficiency and background suppression, which allows us to investigate nuclear reactions with production rates smaller than one atom per week.
"For these experiments, the strong magnetic fields in the BGS focused the element 118 ions and separated them from all of the interfering reaction products which were produced in much larger quantities."
Another important factor for the experiment's success was the unique ability of the 88-Inch Cyclotron to accelerate neutron-rich isotopes such as krypton-86 to high-energy and high-intensity beams with an average current of approximately two trillion ions per second.
"The 88-Inch Cyclotron is the only accelerator in the United States at this time that can provide krypton beams at the intensities that this experiment demanded," said Claude Lyneis, the NSD physicist who heads the accelerator facility for Berkeley Lab.
In operation since 1961, the cyclotron has been upgraded with the addition of high-performance ion sources and can now accelerate beams of ions as light as hydrogen or as heavy as uranium. Today, the 88-Inch Cyclotron is a national user facility serving researchers from around the world for basic and applied studies.
Said I-Yang Lee, scientific director at the 88-Inch Cyclotron, "From the discovery of these two new superheavy elements, it is now clear that the island of stability can be reached. Additionally, similar reactions can be used to produce other elements and isotopes, providing a rich new region for the study of nuclear and even chemical properties."
The Berkeley Lab team plans to repeat their experiment using a target of bismuth rather than lead in an effort to produce elements 117, 115, and 113. Team members have also discussed bombarding a lead target with a beam of ruthenium ions in an effort to reach element 126. At least one theoretical prediction holds that element 126 would be sufficiently stable for doing chemistry. Experiments are already underway at Germany's Society for Heavy Ion Research (GSI) in Darmstadt to confirm the element 118 results.
It is the prerogative of the discovery team to name their element, and Hoffman told the New York Times she intends to propose the name "ghiorsium." Ghiorso has been the codiscoverer of at least a dozen elements, including seaborgium. end
|

