 |
Close Call Underscores Need for Chemical Safety
|
|
|
|
 |
 |
|
|
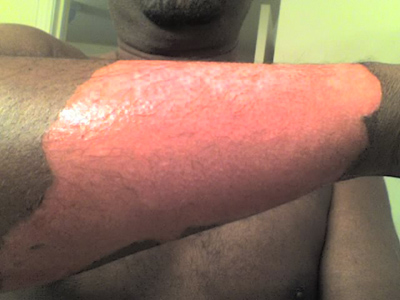
| This burn, which didn't occur at Berkeley Lab, resulted from contact with a half-and-half solution of phenolic acid and formaldehyde, a common solution used in DNA extraction |
|
|
|
Chemical burns, both caustic and acid, cause coagulation of skin proteins and a loss of skin surrounded by an area of poor blood flow. Treatment removes the chemical. Some chemicals require special treatment, such as calcium gluconate gel for hydrofluoric acid burns. Long-term results of burns can include scarring, pigmentation changes, and loss of limb function. Fatalities from inhalation of acids have been reported.
Near-Miss Incident:
Recently, a UC Berkeley student guest decided to etch a germanium wafer in a mixture of nitric and hydrofluoric acids in a fume hood in building 2. He had not performed this operation previously, but had received clear verbal instructions from another member of the research group.
The resulting reaction was more aggressive than he expected. He closed the hood and waited until the reaction had slowed before reopening the hood to recover the sample. When he opened the sash he smelled a strong odor of acid and felt something warm on his cheek.
Aware of the unique toxicity of hydrofluoric acid as a result of attending Chemical Hygiene training, he sought the help of another student in the group, applied calcium gluconate as a first aid measure, and dialed 7911 to summon the Fire Department paramedics -- exactly as he had been trained. As a precautionary measure, the Fire Department transported the student to Alta Bates Hospital for further evaluation. Alta Bates determined that he suffered no injury and released him without treatment. A follow-up evaluation by the EH&S Division and Facilities Division determined that the fume hood was operating within normal tolerances.
Acid-use safety precautions:
- If possible, minimize the amount and/or concentration of acid. Use a substitute for hydrofluoric acid if possible.
- Understand hazards and controls of acids. This information is available from Material Safety Data Sheets and the Chemical Hygiene and Safety Plan. The CHSP has a section on Controls for Acids and Bases. Contact the Industrial Hygienist who supports your Division if you have any questions.
- Supervisors: Ensure that employees can conduct procedures safely and that they know what to expect during the progress of a reaction (e.g., heat generation, production of smoke/fumes and splattering). This may be done by one on one training, demonstration, or other means. In addition, personnel who handle chemicals are required to take Chemical Hygiene and Safety Training (EHS 348).
- Ensure the following emergency equipment is in the area:
- Emergency eyewash and safety shower,
- Hydrofluoric acid exposure kit (available through Health Services at ext 6266)
- Acid spill kit such as JT Baker Safe-T-Spill (available through VWR - Catalog number - JT4442-2).
- Note: hydrofluoric acid requires a special spill kit such as Mallinckrodt "Spill Tamer" HF Acid spill kit (available through VWR - Catalog number MKH35501.
- Conduct operations that may produce an airborne hazard in a fume hood. Keep the sash down to the lowest possible position to control emissions and splashes.
- Wear the following minimum personal protective equipment (PPE) when working with acids:
- Safety glasses with side shields
- Chemically resistant gloves such as Ansell “Sol-Vex” Nitrile gloves for hydrofluoric acid (see “Gloves” in the CHSP for selection guidelines. The section entitled “Controls for Acids and Bases” has additional information for hydrofluoric acid). Remove gloves prior using the phone or computer and before touching door knobs.
- Lab coat
- Supplement with the following PPE, if there is a greater likelihood of exposure from splashes, splattering or fume production:
- Chemical goggles
- Face Shield
- Chemically resistant apron
- Clean up drips and residues from work surfaces and containers.
- Store acids in a corrosive storage cabinet. Use drip trays to contain leaks and spills. Segregate oxidizing acids from flammable and combustible liquids and reducing agents. Segregate all acids from incompatible materials such as bases, reactive metals and chemicals that can produce an airborne toxic hazard (e.g., cyanides and sulfides).
|
 |

