From Basic Physics to Biomedicine
 |
|
| Angelo Bifone made the journey from basic materials science to biomedicine. | |
Lighting up platinum in the lattice
"While I was at the Normale I read about Alex Pines using xenon NMR to study surfaces," Bifone says. "At that time I was interested in the properties of small metal clusters, aggregates of a few tens of metal atoms deposited on surfaces or embedded in porous materials."
The central question was whether these tiny aggregates retained the metallic properties of the bulk metal-for example, whether they would retain large electron conductivity-or would behave like insulators, as theory predicted for particles that were small enough.
"A size-induced metal/insulator transition should dramatically affect cluster surfaces, because for small particles most of the atoms are on the surface. Xenon NMR seemed ideal to me-because of its sensitivity to the surface properties of materials, and because xenon is inert and would not chemically react with the metal cluster."
 |
|
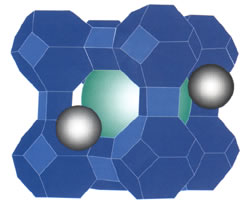
| Clusters of about 55 platinum atoms are trapped in the uniform pores of a zeolite. Xenon atoms, used in NMR studies, reveal the surface properties of the small metal clusters | |
When Bifone applied to finish his Ph.D. work in Pines's lab, Pines not only agreed but also arranged the necessary financial support. There Bifone studied clusters of platinum atoms, each with about 55 atoms and only a nanometer across. By trapping the platinum inside the small pores of a zeolite-a mineral with a regular lattice structure-uniform cluster size would be assured.
At very low temperatures, the xenon stayed on the surface of the metal cluster and was kept from rapidly diffusing and smearing the NMR signal. In this way Bifone could study highly localized surface sites with different geometries, electronic densities, and chemical reactivities-effectively "lighting up" the molecular landscape with xenon NMR.
Bifone, Pines, and their colleagues observed distinct differences in the NMR signal, depending on where the xenon atoms contacted the surface of the cluster. "An unexpected result was that there were both metallic and nonmetallic sites on the platinum," Bifone says, "which shows that the metal/insulator transition is not abrupt."
 |
|
| In 1995 Angelo Bifone received his doctorate from the Scuola Normale Superiore in a ceremony attended by his senior advisors. | |
This discovery and other findings formed the basis of Bifone's Ph.D. dissertation, as well as a letter published in Physical Review Letters early in 1995. That same spring, Alex Pines traveled to Pisa to be present for the ceremony at which Bifone was granted his doctorate.
Pinenuts in pursuit
Meanwhile, Pines and his "Pinenuts"-the graduate students, postdoctoral
fellows, and staff members whom he credits for much of his laboratory's
success-had been pursuing a number of other uses for hyperpolarized xenon.
Bifone, by now a seasoned Pinenut, found himself in the thick of these
discoveries.
"It appeared that the amazing sensitivity of xenon NMR-until now
applied to the study of materials-could be used to study biological systems,"
says Bifone. Although other researchers had done MRI of the lungs and
brains of subjects who had inhaled hyperpolarized helium and xenon gas,
"to Alex it was clear that xenon-a proven, safe medical anesthetic-would
also be sensitive to the chemistry of biological
systems."
 |
|
| NMR and MRI sensitivity is vastly increased by spin polarization-using a laser beam to increase the proportion of spin-up nuclei in a gas such as xenon. Hyperpolarized-xenon techniques developed in the Pines laboratory were featured in the July, 1999, issue of Spectroscopy. | |
In collaboration with Thomas Budinger of Berkeley Lab's Life Sciences Division, Pines, Bifone, and their teammates studied methods of introducing laser-polarized xenon into human blood and tissues. The catch was that xenon's response in biological systems wanes rapidly, as its spin polarization is damped by oxygen and other molecules. Bifone became intrigued by the problem of introducing laser-polarized xenon into the body in ways that would allow it to maintain its high degree of polarization.
The team found that by dissolving the hyperpolarized gas in fluids such as saline solution or perfluorocarbon (which is used as a blood substitute) before introducing it into the bloodstream, NMR signals could be detected for up to 13 seconds, several seconds longer than polarized xenon introduced into the blood through respiration. In a different approach, they trapped the polarized xenon inside lipid vesicles, tiny biochemical balloons that kept the gas from interacting with blood and could be used to carry the xenon through the bloodstream to a targeted organ or tissue.
"By injecting polarized xenon in solution we were able to obtain strong NMR and MRI signals, which allowed us to observe xenon penetrating red blood cells for the first time," Bifone says. The achievement was reported in an article in the Proceedings of the National Academy of Sciences late in 1996 and formed the basis of a patent application.
Before long the technique had been extended to imaging a variety of specific physiological structures including the brain, liver, and muscles, and was soon licensed to Nycomed Amersham, a world leader in diagnostic medical imaging. John Padfield, head of the company's imaging division, notes that the technology "has the potential to revolutionize the way physicians image organ function, and ultimately the way they diagnose illnesses."
Targeting tumors
Possible biological applications of hyperpolarized xenon had also attracted
the attention of medical institutions, including the University of London's
Institute of Cancer Research (ICR) in England. Soon after giving a talk
there on his ideas for using NMR to measure cancerous tumors, Bifone was
invited to join the staff as a lecturer.
The ICR is associated with the teaching hospital of the Royal Marsden National Health Service Trust, and together they form one of the largest centers in the world dedicated to translating basic research directly into patient care and treatment. One of the most challenging problems facing cancer diagnosis and treatment is to understand the vascularization of tumors.
"Rapidly dividing tumor cells need oxygen," Bifone explains.
"They secrete blood-vessel growth factors, which promote a form of
development different from the vascularization of normal tissues."
A tangled and inefficient network of blood vessels sprouts throughout
the tumor as it grows. "One result is that some regions of the tumor
are hypoxic-starved for oxygen. Paradoxically, these regions are more
resistant to radiotherapy and chemotherapy, so it's important to identify
them."
While imaging methods such as PET scans have been used to study tumor
perfusion (blood flow) and vascularization, they have limited spatial
resolution and are not sensitive to the oxygenation level of the tumors.
Measuring tumor oxygenation often involves such invasive procedures as
inserting electrodes.
Hyperpolarized xenon, however, if dissolved in a medium such as perfluorocarbon, is an excellent way of tracing the perfusion of blood through the vascular network of a tumor using MRI. In addition, xenon NMR is highly sensitive to oxygen in the blood and tissues.
 |
|
| Angelo Bifone and Alex Pines discuss the interpretation of NMR and MRI experiments using a high-field-strength magnet (rear) in Pines's laboratory. | |
Bifone explains that "xenon doesn't detect oxygen directly but binds to specific cavities in hemoglobin," the protein that transports oxygen and carbon dioxide in the blood. "Oxygen changes the conformation of hemoglobin and reduces the degree of xenon binding."
At ICR, Bifone and his colleagues are developing methods to exploit xenon's sensitivity to the presence of oxygen, both to map hypoxic areas of tumors and for studies of oxygen concentration in regions of the brain as well, work that has already resulted in several publications. Their research benefits from Bifone's continued close ties with Alex Pines.
Out of curiosity, progress
"My work at ICR in extending the applications of hyperpolarized-xenon
NMR to biomedicine is a straightforward development of previous work with
Alex," Bifone says. "I've visited him several times, sometimes
for long periods, and he's shared his ideas and methods. He's very open."
Says Pines, "The world of science is very international; it is both competitive and cooperative. There's a common language that asks, what does this mean? How does this work? What are the scientific principles that dictate it? Because these questions are universal, there is a shared enthusiasm that tends to forge friendships."
Science crosses not only international boundaries but the artificial boundaries imposed by departments and disciplines as well-although Angelo Bifone looks at his cross-disciplinary career so far with bemusement.
"It's amazing that xenon's physical and chemical properties make
it sensitive to the surface states of metal clusters and also make it
a powerful probe for blood and tissue oxygenation," he says. "When
I went off to Pisa to be a physicist, I never imagined that research based
purely on curiosity about nature could lead to applications that might
have a direct impact on people's lives."
Magnetic Insights
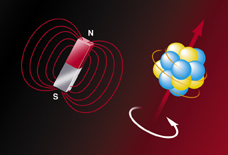
NMR depends on the fact that the nuclei of some atoms have a quantized property called spin, which gives rise to a tiny magnetic moment. In a strong magnetic field, these miniature magnets attempt to line up along the field lines-either along the applied field, "spin up," or in the opposite direction, "spin down." Because spin-up nuclei slightly outnumber more energetic spin-down nuclei, the net effect is a weak overall magnetization.
If, says Alex Pines, "the nuclear spins are subjected to pulses of radio waves at the frequencies corresponding to the up-versus-down energy differences, they are neither up nor down, but in a superposition, a peculiarly quantum combination of the two basic states."
In classical terms, it's as if their spins cause the nuclei to precess-to wobble on a tilted axis around the field lines. Each element has its characteristic wobble rate: the single proton of a hydrogen nucleus precesses four times faster than a nucleus of carbon 13, for example.
 |
|
| Some spinning nuclei have magnetic moments-not unlike a toy bar magnet-and orient themselves with the field lines of an external magnetic field. | |
When a system of nuclear spins returns to its equilibrium state, it reemits the radio energy it absorbed as a detectable NMR signal. Like a telltale fingerprint, the resonance frequency of this signal is a giveaway signature of the chemical species of the nuclei; it carries information about both the relative positions of atoms and their chemical environment.
Xenon gas is particularly useful in NMR studies because the spins of its nuclei are readily polarized by a process called "optical pumping." When a circularly polarized beam of laser light is shone through a mixture of rubidium vapor and xenon, the spin of the photons is transferred first to the rubidium atoms and thence to the xenon atoms.
Instead of just a slight majority of spin-up nuclei, which even at the highest magnetic fields available in the laboratory is normally only one in 100,000 (.00001 percent), in "hyperpolarized" xenon there are as many as 20 percent or more, yielding a much stronger NMR signal. Moreover, this spin can be transferred to atoms the xenon comes in contact with, enhancing their NMR signals as well and leaving an indelible NMR fingerprint. - end -
| < Research Review | Top ^ | Next > |