 |
|
| Eduardo Saiz (left) and Antoni Tomsia pictured in their laboratory. | |
Now, a biologically active glass that enables metal implants to bond with bone could significantly extend the lifetime of artificial hips, knees, and other medical reconstructive devices.
Most implants today are made from either titanium-based alloys or alloys made from a mix of cobalt and chromium. Both possess excellent mechanical properties, but neither is able to bond with bone. As a result, these metals rub against the bones into which they've been implanted, creating wear and tear that shortens implant lifetimes. For example, according to the National Center for Health Statistics, hip implants generally fail after 15 years, and one out of every five hip-replacement surgeries is performed to replace one of these failed implants.
"What has been needed is a coating that adheres to the metal surface of the implant and also promotes the formation of hydroxyapatite (the inorganic component of bone)," says Antoni Tomsia, a materials scientist with Berkeley Lab's Materials Sciences Division, which has maintained a long-term program for the study of ceramic/metal interfaces. Working with physicist Eduardo Saiz, Tomsia has developed a silicate glass that is bioactive and a simple "enameling procedure" whereby metal implants can be coated with micron-sized (20-200 microns thick) layers of this glass. These glass layers can be fine-tuned at the metal-glass and glass-bone interfaces so that the coating binds with both metal and bone.
"It is impossible to design a single coating that will serve all purposes, so what we have done is to create a set of two to three graded layers of coating," says Tomsia. "The glass is cheap to make and the enameling is inexpensive."
In the enameling process used by Tomsia and Saiz, precursor powders are painted on the metal surface and the implant is then annealed at temperatures of 800 to 900 degrees Celsius. Titanium and cobalt-chromium remain solid at these temperatures but the glass becomes liquid. The liquid glass will uniformly coat the surface of even the most intricately shaped implant, a marked advantage over plasma coatings that must be sprayed onto a surface.
 |
||
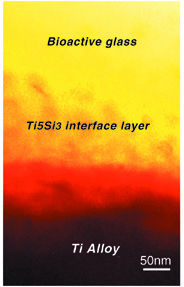
| New bioactive glass can be tuned to the same thermal expansion coefficient as the titanium alloy it coats, avoiding the generation of thermal stresses. Processing at 800�C results in a 100-nanometer-thick adhesive interface layer between the glass coating and the alloy. | ||
There have been previous tests of ceramic coatings on metal implant surfaces, but the rates at which the ceramics and metals expanded under the heat of processing differed so much that large stresses were introduced. These thermal stresses led to cracks in the ceramics at the metal interface which weakened the adhesion between the two surfaces. Furthermore, chemical reactions between the ceramic and the metal, particularly titanium, weakened the implant.
"We can adjust our coatings so that the thermal coefficient of the glass matches that of the metal alloy," says Tomsia. "This eliminates the introduction of thermal stresses during processing."
Tests with titanium and cobalt-chromium showed that the inner surface of the bioactive glass coating of Tomsia and Saiz adheres to the metal without degradation. Upon exposure to simulated body fluid during in vitro testing, a layer of hydroxyapatite will form on the coating's outer surface.
"Implants that are more durable and longer lasting will promote faster healing rates and should be accessible to a wider range of patients," says Tomsia. Over the next year, he and Saiz will be extending their studies to in vivo testing on animal models. This research was funded by the National Institute for Dental and Craniofacial Research of the National Institutes of Health. - end -
| < Research Review | Top ^ | Next > |