 |
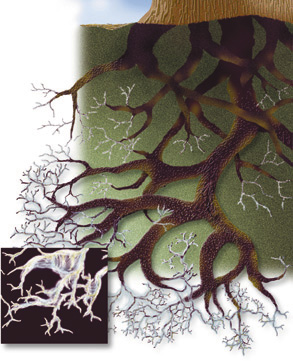
| Beneath the surface of a Norwegian forest, the tips of the tree roots are capped by threadlike filaments of symbiotic fungi which provide nutrients, regulate moisture-and protect the trees from metal poisoning. |
Forest Murmurs
On a warm summer day in southern Norway, where sunlight dapples a forest floor cushioned with needles, the fragrant pine and spruce give no indication that for two decades a constant rain of zinc, copper, lead, and cadmium compounds has settled into the soil-waste from refineries in the industrialized regions east of the Baltic Sea. With poison falling from the air for so long, why are the trees still healthy? The answer lies under the soft carpet of needles, just beneath the surface of the soil. There, the tips of tree roots are penetrated and capped by threadlike filaments of fungi known as ectomycorrhizae. Like the vast majority of terrestrial plants, the Norwegian conifers depend upon these symbiotic fungi to regulate their water supply and provide essential nutrients; in return, the trees feed the fungi with sugars. "There are hundreds of species of ectomycorrhizae in the Scandinavian forests, and each kind of tree has its own," says Geraldine Lamble of Earth Sciences, an x-ray spectroscopist at the Advanced Light Source (ALS) and a member of the Center for Environmental Biotechnology (CEB). "Some grow into connected mats that extend for meters, linking trees with one another to form a network for sharing nutrients." Using ALS beamline 10.3.2, Lamble has studied samples of fungi supplied by researchers at the Norwegian University of Science and Technology. With x-ray fluorescence microscopy and x-ray absorption fine-structure spectroscopy (XAFS), she and her colleagues have identified some of the ways in which ectomycorrhizae isolate heavy metals and keep them from poisoning the trees. "We located a region of the live fungus only 400 by 400 microns in area (a micron is a millionth of a meter) where fluorescence revealed the presence of zinc," says Lamble. Graphs of x-ray absorption at electron orbitals, such as an atom's innermost K orbital, show steep rises called edges; edge shapes and positions are specific to individual elements and molecular bonds. "When we scanned the beam over the zinc K edge, we could see that the zinc was concentrated in regions only a few microns across. The shape of the K edge in the fungus sample matches the very distinctive standard graph for zinc oxalate."
 |
| Geraldine Lamble, an x-ray
spectroscopist at the ALS. |
Down in the Farm
 |
|
|
|
Coenocytic fungi, multi-celled human beings, and other complex creatures are the minority representatives of life on Earth, vastly outnumbered by hundreds of thousands, perhaps tens of millions, of species of simpler microorganisms. When it comes to cleaning up leftovers, their versatility matches their ubiquity. "There is no known compound, artificial or natural, that bacteria can't break down-eventually," says CEB's Terry Hazen, a microbiologist who is the head of the Earth Sciences Division's Ecology Department and the lead scientist in the Environmental Remediation Technology Program. "Eventually can be a very long time, however. The public perception that bioremediation is a natural process that can clean up any contaminant, anywhere, is such a gross oversimplification that it amounts to a hoax." Hazen stresses that every clean-up problem poses a unique set of challenges, including the acidity, salinity, and soil conditions of the subsurface environment as well as the nature of the contaminant itself. Hydrocarbons and other toxic chemicals, loaded with the sustenace of life, can often be metabolized completely to harmless gases; metals must be transformed to less toxic species. Some contaminants must be physically isolated from their surroundings, while others have to be dispersed. Some can be treated in situ, while at other sites the soil has to be excavated and treated in prepared beds. Aeration, moisture, additional con- taminants, and gas emissions may have to be controlled using techniques with such expressive names as bioventing, biosparging and bioslurping. In some sites, nutrients can be added to help local microbes do the job (biostimulation), or special organisms can be brought in (bioaugmentation), or both. In a few cases, it's enough to make sure that nature takes its course. Where the ground formation is permeable, where the contaminant is a good source of carbon (such as spilled fuel or chlorinated solvents) and where the indigenous organisms are well adapted, biostimulation may offer the best choice of technologies. In methane-enhanced biostimulation, for example, pulses of natural gas are injected near the contaminant plume, causing indigenous organisms to multiply; each time the influx of nutrients is turned off, a much larger colony of hungry microbes needs food and begins eating the contaminant. Of such elegant methods of bioremediation, Hazen remarks, "It's really just adding fertilizer-a form of farming."