|
This motor pulls with about 57 to 60 piconewtons of force
which, scaled up to human dimensions, would be enough to lift
six aircraft carriers," says Bustamante, a biophysicist
who, in addition to his affiliation with Berkeley Lab, also
holds appointments with UC Berkeley and the Howard Hughes
Medical Institute.
Biomolecular motors are proteins that undergo shape changes
in order to generate force or torque. Acting like tiny engines,
biomolecular motors come in a wide assortment of varieties
and perform a broad range of tasks, many involving movement
and transportation. One such task is the packing of coiled
lengths of DNA into the protective external shell or "capsid"
that is prominent on a number of viruses including those that
cause herpes, chicken pox and shingles. The biomolecular motor
that Bustamante and his colleagues observed is the portal
motor for the bacteriophage ø29 (phi-29), a virus that
infects and destroys soil bacteria, and is considered an excellent
model system for studying viral assembly.
|
| "This motor
pulls with about 57 to 60 piconewtons of force which,
scaled up to human dimensions, would be enough to lift
six aircraft carriers." |
|
"The portal motor for bacteriophage ø29 compresses
the DNA into a space that is 6,000 times smaller than its
normal volume," says Bustamante. "This generates
an internal pressure of about 60 atmospheres, which is about
ten times that in a champagne bottle."
Bustamante and his collaborators propose that just as the
internal pressure in a
champagne bottle will pop a champagne cork, so too does the
even greater internal pressure inside the bacteriophage's
capsid forcibly pop the viral DNA into an attacked cell. Viruses
cannot "live" or reproduce without getting inside
a living cell, whether it's a plant, animal, or a bacterium.
In the case of ø29, the bacteriophage attaches itself
to and injects its DNA into a soil bacterium, which, unlike
the virus, can reproduce on its own. The viral DNA takes over
the bacterium's reproductive programming and instructs it
to reproduce copies of the virus instead. So many copies of
ø29 are replicated that the bacterium ultimately bursts
open, unleashing a mass of new ø29 viruses ready to
infect other bacteria.
|
|
 |
|
| |
 |
| Biophysicist Carlos Bustamante
with the optical tweezers setup used to measure the strength
of bacteriophage ø29's portal motor. |
|
|
|
"Understanding how this DNA packing process works could
help us design better drugs to interfere with the packing
part of the infection cycle of the virus and perhaps halt
infection," Bustamante says. "It might also be used
in gene therapy as a means of transporting new genetic material
into cells."
To measure the strength of bacteriophage ø29's portal
motor, Bustamante and his collaborators used force-measuring
optical tweezers. Working with capsids that were only partially
packed with DNA before the packing process was stalled, they
tethered the unpacked end of the DNA and the capsid into which
it was being packed between a pair of micron-sized polystyrene
beads. While the capsid-attached bead was held in place by
a pipette, the DNA-attached bead was captured by the optical
tweezers-a laser beam that can be used to grasp and move the
beads.
|
|
| |
 |
 |
|
| |
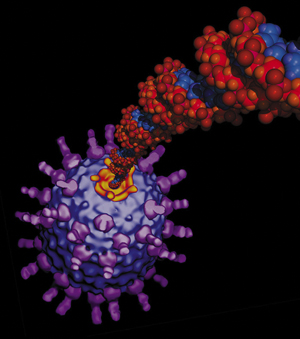
The ø29 motor (yellow)
compresses coiled lengths of DNA into the viral capsid
to 6,000 times its normal volume, creating pressure 10
times as powerful as that inside a champagne bottle. |
|
|
In the presence of adenosine triphosphate (ATP), the fuel
that powers many biomolecular motors, Bustamante and his collaborators
were able to observe viral DNA-packing activity in real time
and measure the force being applied by bacteriophage ø29's
biomolecular motor. This enabled them to calculate the total
amount of work involved, the total internal pressure on the
DNA, and the amount of potential energy available for ejecting
the DNA out of the capsid and into a bacterium during infection.
"The 57 to 60 piconewtons we calculated as the maximum
pull exerted by this motor is an enormous force," Bustamante
says. "The question is then, what happens to all the
work done on the DNA during packing? We claim the energy gets
stored up inside the head of the bacteriophage and becomes
available to initiate rapid injection of the DNA during the
next infection phase."
Collaborating with Bustamante on this research were Doug Smith,
now with UC San Diego, and Sander Tans, now at the Institute
for Atomic and Molecular Physics in Amsterdam, along with
UC Berkeley's Steven Smith, and Shelley Grimes and Dwight
Anderson of the University of Minnesota.
"I would like to emphasize the close collaboration
between my laboratory and that of Dwight Anderson that made
possible this work," says Bustamante. "It was only
because of the excellent complementary expertise of the two
laboratories that this phase of the work was successfully
completed."
The work was funded by DOE Office of Science, the National
Institutes of Health, and the National Science Foundation.
-- Lynn Yarris
|



