Activating Alkanes
 Researchers in LBL's Chemical Sciences Division (CSD)
took an important step
towards harnessing the vast potential of one of nature's most plentiful
materials when they determined how metals can chemically interact with the
large class of naturally occurring hydrocarbons known as alkanes.
Researchers in LBL's Chemical Sciences Division (CSD)
took an important step
towards harnessing the vast potential of one of nature's most plentiful
materials when they determined how metals can chemically interact with the
large class of naturally occurring hydrocarbons known as alkanes.
Alkanes are compounds of carbon and hydrogen atoms held together by single
bonds. The simplest and most abundant is methane, the primary constituent of
natural gas. Chemists have long coveted the use of alkanes as feedstock for
clean-burning fuels and a host of petrochemicals, including plastics, solvents,
synthetic fibers, and pharmaceutical drugs. The problem has been that the bonds
between an alkane's hydrogen and carbon atoms are so strong as to render
alkanes generally unreactive.
CSD researchers, led by chemist and UC
Berkeley professor Robert Bergman, winner of an E.O. Lawrence Award in 1993 for
his alkane studies, have been working with organometallic complexes that can
break carbon-hydrogen (C-H) bonds in alkanes and insert a metal atom (the
metals that they have used so far are iridium, rhodium and rhenium). This leads
to the formation of carbon-metal-hydrogen complexes that are much more
chemically reactive than alkanes and better able to be converted into products.
Utilizing liquefied krypton and xenon as solvents so that the C-H
activation process could be carried out at low temperatures (to slow the
process down), Bergman and his group discovered that metal noble gas and metal
alkane "solvate" complexes were formed as intermediates in the activating
reaction. These weakly bound "alkane complexes" are formed before the C-H
bonds are broken.
By combining the data obtained in their liquefied
noble gas experiments, with gas phase data obtained in experiments conducted by
LBL-UCB chemist Brad Moore and his group of researchers, Bergman and his group
have been able to put together a unified picture of the C-H activation process.
This picture shows that larger alkane molecules, such as cyclohexane, bind more
strongly to the metal center in the solvate than smaller alkanes, such as
ethane. This size-binding effect may in part explain why it is so difficult to
activate methane, the smallest but most important alkane. Understanding the
factors behind this difficulty could provide a solution for activating methane
and converting it into useful products.
This past year, Bergman and his
group were also able to measure the energy barrier that must be crossed in
order for the C-H bond-breaking reaction to occur in the alkane complex. The
energy barrier was found to be approximately 5 kilocalories per mole.
-- Lynn Yarris
 Researchers in LBL's Chemical Sciences Division (CSD)
took an important step
towards harnessing the vast potential of one of nature's most plentiful
materials when they determined how metals can chemically interact with the
large class of naturally occurring hydrocarbons known as alkanes.
Researchers in LBL's Chemical Sciences Division (CSD)
took an important step
towards harnessing the vast potential of one of nature's most plentiful
materials when they determined how metals can chemically interact with the
large class of naturally occurring hydrocarbons known as alkanes. 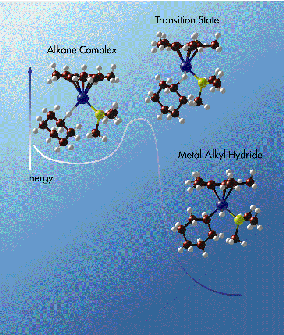
 Return to Highlights Table of Contents
Return to Highlights Table of Contents