

Mention the term "energy research" and the image most likely to be conjured is that of massive power plants or sprawling factories. Although LBL does investigate ways of improving the efficiency and reducing the pollution of such plants, its scientists are also conducting research into areas less dramatic but no less important. For example, consider the act of switching on a light. American taxpayers annually spend an estimated $30 billion in energy costs for electrical lighting--including residential, commercial, industrial and street lighting, plus lighting for miscellaneous purposes such as stadium events. This accounts for about 25 percent of the electrical energy consumed in the U.S each year. Scientists at LBL believe this consumption could be cut in half if existing lighting systems were to be replaced with new energy-efficient illumination. To this end, they have been working with industry to improve the illumination and energy-efficiency of fluorescent bulbs as a marketable replacement for energy-wasting incandescent bulbs. This past year, however, saw the unveiling of an even newer technology that has the potential to rewrite the future course of electrical lighting. Scientists call this promising new technology the "molecular emitter lamp," but it will probably come to be known to consumers as the "sulfur lamp."
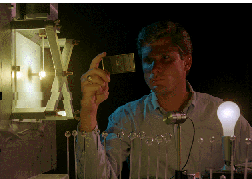 Doug Crawford and his colleagues at LBL's Lighting Lab helped a commercial company
develop this energy saving ultra-bright sulfur lamp.
Doug Crawford and his colleagues at LBL's Lighting Lab helped a commercial company
develop this energy saving ultra-bright sulfur lamp.
Four times more energy efficient and 700 times brighter than a conventional
incandescent bulb, the sulfur lamp also outperforms the best of the new
fluorescent bulbs. The first lamp, produced in conjunction with Fusion Lighting
Inc. of Rockville, Maryland, consisted of a glass globe the size of a golf ball
that was filled with argon gas and a tiny amount of non-toxic sulfur, rather
than the toxic mercury gas used in fluorescent tubes. Microwaves were used to
electrically charge the argon gas which in turn heated the sulfur and made it
glow, giving off a rich bright light that was described as akin to having a
miniature sun in the room. A prototype version of the lamp is now on public
display in Washington D.C. at both the U.S. Department of Energy's headquarters
and at the Smithsonian Institution's National Air and Space Museum.
Equally important to national energy conservation efforts are energy-efficient windows. Each year, the amount of money required to offset the heat lost in the winter and gained in the summer through windows costs American taxpayers about $25 billion--a loss comparable, in terms of energy, to the amount of oil delivered through the Alaska pipeline. LBL scientists, through such projects as "superwindows" and the new and improved "smartwindows," have been transforming windows from an energy liability to an energy asset.
In addition to research on lights and windows, LBL scientists also develop computer models that simulate all aspects of energy consumption inside buildings. Given that Americans spend more than 80-percent of their time indoors and that more than $200 billion of this country's yearly energy bill is spent on meeting indoor energy needs, the potential for savings is substantial. This past year, LBL energy researchers released a new and even better version of "DOE-2," one of their most successful simulation programs. This new version, called "DOE-2.1E," like its predecessor is a tool to help engineers and architects reduce energy consumption in the buildings they design or retrofit. The new program can calculate the hourly energy use and energy costs of any type of commercial or residential building over a year's time based on such information as the dimensions, construction materials, geographic location and orientation of the building, plus local weather data. If given the local utility rates, the program will even calculate the building's annual energy bill. With DOE-2.1E, the energy-efficiency of alternative building designs can be compared and optimized long before any concrete is poured.
LBL energy researchers have not ignored the larger problem of factory waste and pollution. The same group of LBL scientists who developed a cost-effective technology for clearing the air of sulfur dioxide pollution from power and chemical plants have now taken a big step toward clearing the water of future pollution from pulp and paper mills. This past year they announced the development of a chemical process called "POZONE" that cuts in half the cost of producing ozone. By putting ozone, which is a molecular form of oxygen that can serve as an environmentally benign bleach, on a cost-competitive footing with chlorine, the POZONE process will help U.S. paper manufacturers meet proposed new EPA standards.
 LBL researchers Ted Chang and Shu-Mei Wang have developed
a chemical process called "POZONE" that cuts in half the cost of producing
ozone.
LBL researchers Ted Chang and Shu-Mei Wang have developed
a chemical process called "POZONE" that cuts in half the cost of producing
ozone.
Paper products are a $125-billion business in the U.S.--the
nation's eighth largest industry. Last year, U.S. paper mills produced about 14
million tons of pulp which they bleached with chlorine to make white paper.
Because chlorine bleaching creates wastewater containing hazardous chemicals,
the EPA wants U.S. mills to find a substitute. In the past, ozone-bleaching has
been considered too costly because the production of ozone involved an
expensive electricity-based process. POZONE produces ozone through the
inexpensive chemical reaction of yellow phosphorus with oxygen. The potential
for POZONE extends well beyond the pulp industry, because the technique can
also be used to recycle carbon that has been used by a large number of
different industries to adsorb toxic solvents. This contaminated carbon has
traditionally been buried, which means it can pose a threat to groundwater.
Removing buried toxic wastes that have been found to be contaminating groundwater has always been difficult. Even assessing the risk posed by these underground sites has proved troublesome. The reason is that both tasks require the drilling of holes into soil that is of such poor quality (which is why it was chosen as a burial site for waste) it often crumbles and clogs up the hole. Conventional drilling muds used to stabilize commercial bore holes can't be used for fear of spreading contaminants even further. LBL earth scientists may have found a solution with the development of a drill that blasts super-cold nitrogen gas as it bores, creating frozen holes that won't collapse even in the most difficult of soils. In this technique, which is called "cryogenic drilling," nitrogen is injected down the drill's center pipe and exits through nozzles near the spinning drill bit. At 196 degrees-below-zero Celsius, the gas freezes difficult soils rich in sand, gravel or ash long enough for workers to insert stabilizing metal casings into the holes before the ground thaws.

Return to the Table of Contents of the 1994 Regents Report
