
Berkeley Lab Scientist Wins E.O. Lawrence Award
Moore
Foundation Boosts Supernova Research
Berkeley Lab Scientist Wins E.O. Lawrence Award
 Richard Saykally, a professor of chemistry with UC Berkeley who joined
Berkeley Lab’s Chemical Sciences Division (CSD) a year ago, has been
named one of seven new winners of the E.O. Lawrence Award by Secretary of
Energy Spencer Abraham. Saykally won the award in the chemistry category
for his groundbreaking developments in the field of spectroscopy.
Richard Saykally, a professor of chemistry with UC Berkeley who joined
Berkeley Lab’s Chemical Sciences Division (CSD) a year ago, has been
named one of seven new winners of the E.O. Lawrence Award by Secretary of
Energy Spencer Abraham. Saykally won the award in the chemistry category
for his groundbreaking developments in the field of spectroscopy.
“We are all enriched by the contributions these researchers have made, ranging from engines with no moving parts to better ways to see the stars,” Secretary Abraham said in announcing the 2004 Lawrence Awards, which were named for Berkeley Lab’s founder, Ernest Orlando Lawrence, the Nobel Prize-winning inventor of the cyclotron. The awards, the highest given by the DOE, recognize outstanding scientific contributions in atomic energy.
“The Lawrence awards, and the research for which they are given, show that DOE could easily be called the Department of Science and Energy,” Secretary Abraham said.
Each winner of this year’s Lawrence Award will receive a gold medal, a citation, and $50,000. The awards will be presented at a ceremony in Washington, D.C. on Nov. 8.
Saykally, a professor on campus for 25 years, is Berkeley Lab’s 26th recipient of a Lawrence Award. His award citation reads: “For the invention of velocity modulation spectroscopy of molecular ions; for the development of far-infrared vibration-rotation spectroscopy of radicals, clusters and carbon chains; for the elucidation of the structure and potential energy surfaces for water clusters; and for the development and application of cavity ring-down spectroscopy techniques.”
The Lawrence Awards were established by Dwight D. Eisenhower in 1959. Recipients are chosen by independent panels from thousands of nominations by international scientists and research organizations. The awards are intended to encourage the careers of scientists who show exceptional promise.
The other 2004 Lawrence Award winners were Nathaniel Fisch of Princeton University and the Princeton Plasma Physics Laboratory; Bette Korber, Fred Mortensen and Gregory W. Swift of Los Alamos National Laboratory; Claire Max of UC Santa Cruz and Lawrence Livermore National Laboratory; and Ivan Schuller of UC San Diego.
Saykally has spent much of his professional career finding unique new ways to explore important physical phenomena we know little about.

Richard Saykally is the 26th Berkeley Lab scientist to win an E.O. Lawrence
Award, the highest DOE science honor.
Take, for example, his invention of velocity modulation spectroscopy to study molecular ions — enigmatic and elusive molecules with a net electrical charge. Molecular ions play critical roles in many areas of chemistry and physics, including the creation of plasmas (ionized gases), such as those found in lightning and the Northern Lights, and the formation of molecules in space and the upper atmosphere. Using standard spectroscopy techniques to study molecular ions is difficult because their light absorption properties are overlapped and drowned out by the stronger light absorption of electrically neutral molecules. Saykally solved this problem through the use of an alternating electric discharge that caused molecular ions to move towards the negative electrodes while neutral molecules remained unaffected. This enabled him to easily distinguish the molecular ions in his spectroscopic studies.
Said CSD Director Dan Neumark, “Rich Saykally is one of the world’s leading spectroscopists. He has invented several new experimental techniques and has developed novel conceptual frameworks for understanding the high-resolution spectra of weakly bound species, such as ammonia and water clusters, which cannot be treated by the standard tools of spectroscopy.”
Born in Rhinelander, Wis., in 1947, Saykally earned his B.S. at the University of Wisconsin at Eau Claire in 1970 and his Ph.D. at the University of Wisconsin at Madison in 1977. Prior to coming to Berkeley in 1979, he was a National Research Council Postdoctoral Fellow at the National Institutes for Science and Technology at Boulder, Colo. He currently holds the Class of 1932 Distinguished Professor Chair at Berkeley and is the co-author of more than 300 scientific publications.
Among the 35-and-counting
scientific honors Saykally has received are the National Science Foundation’s Presidential Young Investigator Award, the E. R. Lippincott Medal for Spectroscopy, the Centenary Medal of the United Kingdom’s Royal Society of Chem-istry, and the Irving Langmuir Award in Chemical Physics. He is a member of the National Academy of Sciences, a Fellow of the Royal Society of Chemistry, the American Physical Society, the Optical Society of America, the American Academy of Arts and Sciences, and the Amer-ican Association for the Advance-ment of Science.
Saykally was also recognized as an outstanding educator when he won the UC Berkeley Distinguished Teach-ing Award in 1992. In his career, he has mentored nearly 50 Ph.D. students as well as a large number of postdoctorals and undergraduates, and has been actively involved in national secondary school education projects.
Upon learning he had won the Lawrence Award, Saykally said, “It is a real honor to follow the many great Berkeley scientists who have been recognized by this award, and it is a fitting tribute to the accomplishments that my brilliant students and postdoctorals have managed through their hard work and creativity.”
Moore Foundation Boosts Supernova Research
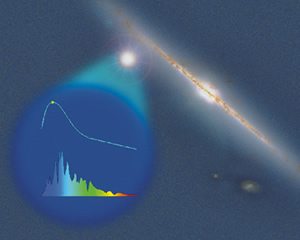
The Nearby Supernova Factory will compile a catalog of the light curves, spectra, and other features of hundreds of nearby Type Ia supernovae.
The federal government has lately paid less attention to the history and fate of the universe than to returning to the moon. Thus a recent $2.38-million grant from the Gordon and Betty Moore Foundation supporting the work of the Nearby Supernova Factory (SNfactory) is particularly welcome. Its purpose is to further dark energy research through the study of Type Ia supernovae.
“We are delighted with the wisdom and foresight shown by the Moore Foundation in supporting cutting-edge research in the physical sciences,” says Saul Perlmutter. He and Michael Levi, both of the Physics Division, are principal investigators for the grant, made to UC Berkeley’s Space Sciences Laboratory. They note that “there was no natural home for this research in the shrinking federal physical research portfolio.”
Because Type Ia supernovae are very bright and remarkably similar in their brightness, they make the best cosmological “standard candles.” In 1998 Perlmutter and his colleagues used them to conclude that the universe is flying apart at an accelerating rate, propelled by mysterious dark energy.
“Good as Type Ia supernovae are as standard candles, there is a residual uncertainty of a few percent in brightness measurements, and thus distance measurements,” says Levi, an uncertainty which must be reduced for futher studies of dark energy.
The way to reduce uncertainty is to learn more about the physics of nearby supernovae. Physics Division astronomer Greg Aldering, who leads the international SNfactory, says, “We designed the SNfactory to discover hundreds of nearby Type Ia supernovae while they are still brightening — and close enough to measure with great precision.”
The Gordon and Betty Moore Foundation was established by Intel cofounder Gordon Moore (best known for “Moore’s Law” predicting the doubling of computer chip capacity every two years) and by his wife Betty. The Foundation supports environmental conservation, science, and higher education.
Autumn Moon Festival Celebrates Asian Traditions


Take one full moon, add 1,000 years of tradition, and you’ll get the Autumn Moon Festival, an occasion celebrated by nearly 90 Berkeley Lab employees and their families last Thursday thanks to the Asian Club.
The festival is a popular celebration of abundance and togetherness dating back to China’s Song Dynasty, more than 1,000 years ago. The event traditionally occurs on the fifteenth day of the eighth lunar month, when the moon is at its fullest and brightest — an ideal time to celebrate the summer harvest and the lore of mythical moon goddess Chang O.
“The Autumn Moon Festival has many themes, but the main one is, it traditionally emphasizes the importance of family and community unity,” said Paul Gee, who was anointed “king” of the event. “We want the Lab to be more aware of Asian culture, and we also want to reflect the diversity of the people who work here.”
A king needs a queen, so Laura Luo was anointed “queen,” and the two presented Lab Director Steven Chu with a moon cake. Attendees also enjoyed Chinese moon cake while listening to Larry Li Guo’s version of “Chang’er the Beauty and Houyi the Archer,” a 1,000-year-old myth about their love for each other, the moon, and moon cakes. Last week’s occasion marked the first time the Asian Club celebrated this tradition.
Ready, Set � Runaround XXVII Next Friday

Twenty-seven years ago, Bruce Heppler pedaled his unique wheeled
contraption amid the throng of runners and walkers in the Lab's
first Runaround.
Whoever said you can’t improve on a good thing must have had the Berkeley Lab Runaround in mind. Now in its 27th year, the event has the same look and feel as the very first time that hundreds of walkers, runners, and riders of various vehicles huffed and puffed their way up and down the Lab’s slopes back in 1978.
When participants take off next Friday, Oct. 8., they will walk, run or bike roughly the same course, partake in the same sense of competitiveness and goofiness, and will be greeted at the cafeteria, as always, with food, music, as well as the highly treasured Runaround t-shirts. Even the funky prize categories that first Runaround organizer Harvey Levy called “the categories of absurdity” will be awarded as always — everything from best legs to best costumes.
The race begins at the Firehouse at noon and ends at the cafeteria parking lot. The course is 1.86 miles (3 kilometers) long, with some steep elevation changes.
All employees and retirees healthy enough to take on the challenge are encouraged to participate. To add to the whimsical atmosphere, individual and group costumes are highly recommended. Needless to say, the light-hearted spirit will not deter the competitiveness of the elite runners, who have their eyes set on crossing the finish line first. The timers are ready for them.
To make sure that all participants in the Runaround have a good time and stay healthy before, during and after the event, organizers and members of the Lab’s Health Services have the following suggestions:
Before the Runaround:
- Check with your doctor to make sure you have no medical problems that may preclude you from taking part.
- Prepare by walking or running three to four times a week to increase strength and endurance.
- Eat a meal composed of protein and carbohydrates two to three hours prior to each workout.
- Drink fluids two to three hours before each workout and bring water to drink during your walk or run.
- Wear comfortable shoes and clothes that do not restrict your stride or rub against your skin.
- Apply sunscreen to your face, ears, neck, arms, and legs.
During the Runaround:
- Prepare your body by doing stretching exercises.
- Carry water to drink.
- Apply sunscreen to sun exposed areas.
After the Runaround:
- Stop by the Health Services table at the finish line if you experience any medical problems.
- Pick up your t-shirt.
- Partake in the refreshments, entertainment, and camaraderie of the Lab’s most popular event of the year.
For more information on the Runaround, a course map, and past results, see the Runaround website at http://cfi.lbl.gov/~derenzo/ runaround/.
‘Dream Beams’ Achieved with Laser Wakefield Acceleration

Members of the L'OASIS group (left to right) are Wim Leemans, Cameron
“Dream beam,” announces the cover of this week’s Nature, and the beam in question, pictured in sinuous waves of blue and black, was created right here at Berkeley Lab — a major technological advance achieved by the L’OASIS group led by Wim Leemans in the Center for Beam Physics of the Accelerator and Fusion Research Division. (L’OASIS stands for Laser Optics and Accelerator Systems Integrated Studies.)
For a quarter of a century physicists have been trying to push charged particles to high energies with devices called laser wakefield accelerators, which offer the possibility of compact, high-energy machines for probing the subatomic world, studying new materials and new technologies, and medical applications. In theory, particles accelerated by the electric fields of laser-driven waves of plasma could reach, in just a few score meters, the high energies attained by miles-long machines that use conventional radio-frequency acceleration.
But while researchers have generated electric fields in plasmas a thousand to ten thousand times greater than in conventional accelerators, these large fields exist only over the short distance the laser pulse remains intense. Typically that’s only a few hundred micrometers (millionths of a meter) for a tightly focused pulse, and the energies of the accelerated particles are so widespread that fewer than one percent have enough punch for scientific applications.
Channeling better beams

Their research makes the cover of the September 30, 2004 issue of Nature.
Now, using a technique called “plasma-channel guiding,” the L’OASIS group has accelerated bunches of electrons numbering several billion electrons each, all within a few percent of the same high energy of more than 80 million electron volts.
“Laser wakefield acceleration works on the principle that by sending a laser pulse through a gas to create a plasma — separating negatively charged electrons from positively charged ions in the gas — some of the free electrons will be carried along in the wake of the plasma wave created by the laser,” Leemans explains. “Imagine that the plasma is the ocean, and the laser pulse is a ship moving through it. The electrons are surfers riding the wave created by the ship’s wake.”
Unfortunately, simply punching a laser pulse through a plume of gas makes for a very short trip. “The acceleration distance is limited to what’s called the Rayleigh length — the length over which the laser remains focused,” says Leemans. “For optimum acceleration, you have to keep the wake going for many Rayleigh lengths, until just before the electrons start to get ahead of the wave and lose energy” — a distance otherwise known as the dephasing length.
To achieve this, Leemans, working with graduate student Cameron Geddes and other colleagues, employed “the plasma analogue of an optical fiber.” The L’OASIS laser first sends an igniter pulse through the gas, to form a sort of “wire” of plasma. A second, heater pulse enters from the side and heats the plasma wire; as the channel expands, it becomes denser at the edges and less dense at the center. Five hundred trillionths of a second later, an intense driver pulse, with peak power approaching 10 trillion watts, plows through the plasma capillary.
“There’s a close analogy between the plasma channel and an actual optical fiber of the kind used to send data,” Leemans says. In the simplest optical fibers, “the glass in the center has a higher index of refraction — meaning that light moves through it more slowly — and the surrounding glass walls have a lower index of refraction.” Thus the wavefront of the light stays flat as it moves through the fiber.
The same is true of the plasma channel, because the plasma is denser at the edges than in the center. “Plasma actually has a lower index of refraction than vacuum, so the wavefront moving through the center of the channel moves slower than at the edges. This flattens the otherwise spherical laser wavefront and extends the distance over which the laser stays intense.”
How is it that the electrons in the bunch are accelerated to nearly the same energy? To analyze their successful experiments, the group collaborated with the Tech-X Corporation of Boulder, Colorado, using the company’s VORPAL plasma simulation code to model their results on supercomputers at NERSC. The most critical factor, they found, lies in matching the long acceleration path to the dephasing length.
The channeling technique not only opens the way to compact accelerators with multiple stages, says Leemans, but it “has already suggested novel sources of radiation” including coherent infrared and terahertz radiation and efficient generation of x-ray pulses measured in quadrillionths of a second.
“High-quality electron beams from a laser wakefield accelerator using plasma-channel guiding,” by Cameron Geddes, Csaba Toth, Jeroen van Tilborg, Eric Esarey, Carl Schroeder, David Bruhwiler, Chet Nieter, John Cary, and Wim Leemans, appears in the September 30, 2004 issue of Nature.
Meet Lab Director Chu at All Hands Reception
 Berkeley Lab employees who have not yet had a chance to meet new Laboratory
Director Steven Chu in person will get the opportunity next Tuesday. From
3 to 4:30 p.m., he will appear at a welcome “all hands” reception
in the cafeteria main dining room. After brief remarks, Director Chu will
be available to answer questions and greet attendees. Light refreshments
will be provided
Berkeley Lab employees who have not yet had a chance to meet new Laboratory
Director Steven Chu in person will get the opportunity next Tuesday. From
3 to 4:30 p.m., he will appear at a welcome “all hands” reception
in the cafeteria main dining room. After brief remarks, Director Chu will
be available to answer questions and greet attendees. Light refreshments
will be provided
What Lies Beneath in Buried Nanolayer Interfaces
By Lynn Yarris
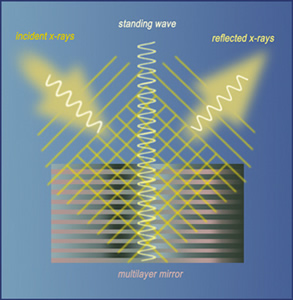
When incident waves of x-rays from the ALS interfere with x-ray waves reflected
from a sample that’s grown on top of a nano-scale multilayer mirror,
a vertical standing wave is formed that can be used to identify and study
various properties of each atom in the sample.
Nanotechnology is superficial, the joke goes, meaning that when objects become so tiny, everything takes place on their surfaces. However, the nanosized components of future computers and memory storage devices will actually consist of multiple overlying layers of materials. This means scientists need a way to selectively study the interfaces buried below surfaces. Researchers at the Advanced Light Source (ALS) have developed just such a technique.
Charles Fadley, a physicist affiliated with Berkeley Lab’s Materials Sciences Division and a professor of physics with the University of California at Davis, led this research effort. His principal collaborators on the project have been See-Hun Yang, now at the IBM Almaden Research Center, and Simon Mun, now an ALS staff scientist.
“We’ve developed a new way of selectively looking below the surface in nanolayers of materials using soft x-ray standing waves,” says Fadley. “This permits us for the first time to apply all of the principal ALS spectroscopies in a much more depth-sensitive way that directly yields chemical state and magnetic information near the buried interface between two materials.”
Layered nanostructures will play an especially critical role in the development of the next generation of magnetic read-heads for high-density data storage, and for the much-anticipated magnetic random-access memory chips or MRAMs. Using spintronics — the spin of electrons rather than their charge — to store data, MRAM promises “instant-on” computers that can store more information and access it faster, while consuming far less power than today’s machines.
To reach these goals, however, scientists and engineers need a way to “see” what’s going on at the interfaces where different layers of materials meet. Furthermore, they need to do this without damaging the materials, a requirement that demands extreme delicacy because each layer is only a few atoms thick.
“The most powerful method for studying these interfaces has been scanning transmission electron microscopy, but it can’t be considered nondestructive,” says Fadley. “Using standing waves of soft x-rays, we can nondestructively analyze buried interfaces for atomic composition, and for chemical, magnetic, and electronic structures.”
 Charles
Fadley (front), working with See-Hun Yang (left) and Bongjin
Simon Mun, are using a standing wave spectroscopy technique
and the circularly polarized light of ALS Beamline 4.0.2 (shown
here) to study buried interfaces in nanolayers of materials
that will be critical to future electronic devices and magnetic
storage media.
Charles
Fadley (front), working with See-Hun Yang (left) and Bongjin
Simon Mun, are using a standing wave spectroscopy technique
and the circularly polarized light of ALS Beamline 4.0.2 (shown
here) to study buried interfaces in nanolayers of materials
that will be critical to future electronic devices and magnetic
storage media.
A standing wave is a vibrational pattern that’s created when two waves of identical frequency interfere with one another while traveling in opposite directions through the same medium. In this case, x-ray waves from the ALS strike a sample grown on top of a nanoscale multilayer mirror, which was designed by researchers at Berkeley Lab’s Center for X-Ray Optics to create especially strong reflected waves.
When incident ALS x-ray waves interfere with waves reflected from the sample, specific points along the interfering waves appear to be standing still, even though they are vibrating up and down. The interactions between these standing waves and the inner “core” electrons of an atom can be used to identify that atom and study its various properties.
Since Fadley and his research group wanted to investigate the thickness-dependence of the phenomena they were studying, they needed to make their standing wave probe depth-sensitive. They accomplished this by growing their samples in the shape of a wedge, and scanning a beam of x-rays across the surface at an angle that created a vertical standing wave.
“The intensity maximum of our standing waves, which generates most of the signal we measure, occurs at a particular depth below the surface,” says Fadley. “This gives us the depth-sensitivity and the high resolution we need to map the changes in chemical and magnetic behavior that take place at, and around, an interface.”
The beams used to create the standing-wave probe are circularly polarized soft x-rays generated at ALS Beamline 4.0.2. A beam of light is circularly polarized when its electric-field component rotates either clockwise or counterclockwise around the direction in which the beam is traveling. The absorption of circularly polarized light by a magnetic material at an interface reveals much about the magnetic moments of the atoms at that interface. This makes Beamline 4.0.2, which is powered by one of the ALS’s premier undulator magnets, ideal for studying the types of buried interfaces that will be found in future data storage devices.
“With soft x-ray standing waves we can, for the first time, look at multilayered nanostructures using a tour de force of all the relevant spectroscopy techniques, including photoelectron, x-ray emission and x-ray absorption spectroscopies,” says Fadley.
Fadley and his colleagues have already used their standing wave technique to gain new insight into the mechanisms behind the phenomenon known as giant magneto-resistance, or GMR — a 20 to 50 percent boost in electrical conductivity that occurs when a magnetic field is applied to interfaces between magnetic and nonmagnetic metals. GMR nanostructures can be found in virtually all of today’s computer disc read heads, and a detailed understanding of the buried interfaces in these devices is critical to their performance.
“We applied the standing wave technique to an iron/chrome interface and were able to determine the width of the interface, as well as a detailed profile of the magnetic field running through it,” Fadley says. “We observed that chrome, which normally is nonmagnetic, was being magnetized by iron just below the interface, but in a direction opposite to the field of the iron.”
Fadley says that the standing wave spectroscopy technique he and his group have developed should also prove useful for future studies of ultrathin films, liquid layers, molecular clusters and environmentally-related surface chemistry. Combined with an x-ray microscope, it could also be used to do spectroscopy in three dimensions
New Insights into Hydrated Electrons
Understanding May Lead to Clues into Biological Processes that Can Cause Disease

Chemical Sciences Division Director Daniel Neumark
Sometimes, it pays to think small. By observing how a single electron behaves amid a cluster of water molecules, a team of scientists has gained a better understanding of a fundamental process that drives a myriad of biological and chemical phenomena, such as the formation of reactive molecules in the body that can cause disease.
The researchers, led by Chemical Sciences Division Director Daniel Neumark, used an extremely fast imaging technique to observe an excited electron, surrounded by several dozen water molecules, relax back to its original energy state. This journey occurred much more quickly than one theory predicts, lending credence to an opposing theory and helping to solve a longstanding puzzle in the world of hydrated electrons.
Neumark conducted the study with scientists from UC Berkeley and Israel’s Tel Aviv University. Their research is published in the September 16, 2004 edition of the online Science Express.
As their name implies, hydrated electrons are electrons that are dissolved in water. They occupy an elliptical void formed by six water molecules, and they’ve intrigued scientists since their discovery in 1962. The simple fact that they exist is interesting, as is their little understood role in many biological and chemical processes. Although it is too early to tell how Neumark’s work will elucidate the behavior of hydrated electrons in the real world, such as how they conspire to form free radicals (highly reactive molecules that can damage tissue and contribute to diseases such as cancer, rheumatoid arthritis, and heart disease), it will help shape future research.
Leading up to this study, scientists had been divided as to how hydrated electrons react after they’ve been excited. One theory holds that electrons convert back to their original energy state in about 50 femtoseconds, or 50 millionths of a billionth of a second. The other theory contends this conversion takes much longer, about 500 femtoseconds.
To help settle the case, Neumark’s team observed a single electron in a tiny cluster of between 25 and 50 water molecules. Such clusters give scientists an extremely close look at the electron’s dynamics. For example, they can determine whether water molecules are simply rearranging themselves around an electron in an excited or a ground state, or whether these dynamics indicate the actual transition of the electron between these states.
The team created an electronic excitation by zapping the cluster with a femtosecond laser pulse. They then used time-resolved photoelectron imaging to take snapshots of the electron as it relaxed back to its ground state. The dynamics and rate of this conversion, when extrapolated to how hydrated electrons behave in bulk, suggest that hydrated electrons relax back to their unexcited state in about 50 femtoseconds — a finding that tips the scales in favor of this theory.
“Resolving which of these two models is correct is a key step. We’ve used time-resolved studies of finite clusters to resolve an issue of fundamental importance, namely the dynamics of an excited hydrated electron,” says Neumark. “More generally, this work represents a fairly unique example of how studies of clusters can elucidate bulk phenomena.
Berkeley Lab Scientists Gain New incite on Photosynthesis
 Seeking to unlock the secrets of photosynthesis are, left to right, Alán
Aspuru-Guzik, Graham Fleming, Romelia Salomón-Ferrer, Brian Austin,
Harsha Vaswani, William Lester, and Ricardo Oliva.
Seeking to unlock the secrets of photosynthesis are, left to right, Alán
Aspuru-Guzik, Graham Fleming, Romelia Salomón-Ferrer, Brian Austin,
Harsha Vaswani, William Lester, and Ricardo Oliva.
Solar power remains the ultimate Olympic gold medal dream of a clean, efficient and sustainable source of energy. The problem has been that in order to replace fossil fuels, we need to get a lot more proficient at harvesting sunlight and converting it into energy. Nature has solved this problem through photosynthesis. All we have to do is emulate it. But first, we need a much better understanding of how photosynthesis works at the molecular and electronic levels.
“After working on the problem for about 3 billion years, nature has achieved an energy transfer efficiency of approximately 97 percent,” says Graham Fleming, director of Berkeley Lab’s Physical Biosciences Division and an internationally acclaimed leader in spectroscopic studies of photosynthetic processes. “If we can get a complete understanding as to how this is done, creating artificial versions of photosynthesis should be possible.”
Towards this end, Fleming has teamed his capabilities in ultrafast spectroscopic experiments with the computational expertise of Chemical Sciences Division theoretical chemist William Lester, Jr. Their collaboration received one of three inaugural INCITE Awards (Innovative and Novel Computational Impact on Theory and Experiment) from the U.S. Department of Energy’s Office of Science. The INCITE program is aimed at advancing a select number of computationally intensive scientific projects with high-impact potential by providing a substantial amount of time on the supercomputers at NERSC.
“The theory behind energy transfer in photosynthesis is more than 50 years old, but it has never been fully tested,” Fleming says. “Some aspects of this theory are beyond current experimental testing capabilities, but can be simulated at NERSC.”
Says Lester, “Before we had computational capabilities such as those at NERSC, it was not possible to model the energy and electron transfer processes we want to study. NERSC can provide us with the computers and software support that will enable us to run codes that will give us the information we need and could not otherwise obtain.”
Life on Earth is dependent upon the photosynthetic reactions that green plants and cyanobacteria use to convert energy from sunlight into chemical energy. Among other things, these reactions are responsible for the production of all of our planet’s oxygen. In high school biology, students learn that nature uses chlorophyll, the family of green pigment molecules, as a light absorber and energy-transfer agent, but the chemistry behind this process is extremely complicated. What’s more, photosynthetic chemistry takes place on a femtosecond timescale (a femtosecond being one millionth of a billionth of a second).
“According to the first law of photosynthetic economics, a photon saved is a photon earned,” Fleming says. “Nature has designed one of the most exquisitely effective systems for harvesting light, with the reactions happening too fast for any light to be wasted as heat. Current synthetic light-harvesting devices, however, aren’t following nature’s model.”
Fleming has been using femtosecond spectroscopy techniques to shed scientific light on nature’s light-harvesting and energy-transferring secrets. Photosynthesis in plants starts with a light harvesting system, which consists of two protein complexes, Photo-system I and Photosystem II. Each complex features light-absorbing antennae made up of members from two families of pigment molecules, chlorophylls and caroten-oids. These pigment antennae are able to capture photons of sunlight over a wide spectral and spatial cross-section.
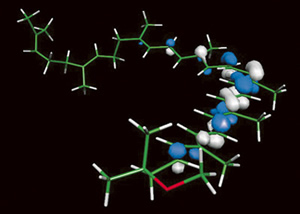 Using
a computational method called a quantum Monte Carlo (QMC) simulation and
the IBM SP computer at NERSC, Berkeley Lab scientists are calculating optimal
electronic pathways for photosynthetic energy transfer. These are the largest
electronic and molecular calculations ever performed for a biological molecule
using a QMC code
Using
a computational method called a quantum Monte Carlo (QMC) simulation and
the IBM SP computer at NERSC, Berkeley Lab scientists are calculating optimal
electronic pathways for photosynthetic energy transfer. These are the largest
electronic and molecular calculations ever performed for a biological molecule
using a QMC code
The chlorophyll and carotenoid molecules gain extra “excitation” energy from the captured photons that is immediately funneled from one neighboring molecule to the next, until it arrives at another molecular complex, which serves as a reaction center for converting energy from solar to chemical. This transferal of excitation energy involves several hundred molecules and hundreds of individual steps along different electronic pathways, yet still transpires within 30 picoseconds for Photosystem I and 200 picoseconds for Photosystem II. By human standards of time, that’s instantaneous.
“If we can follow the steps in transferring energy from donor to acceptor molecules, we might be able to design new and much more effective strategies for synthetic light harvesters,” Fleming says.
Because the extra energy being transferred from one molecule to the next changes the way each molecule absorbs and emits light, the flow of energy can be spectroscopically followed. However, to do this, Fleming and his experimental research team need to know what spectroscopic signals they should be looking for. This is where the INCITE grant will help. Working with NERSC staff members — most prominently, David Skinner — and using NERSC’s IBM SP computer, Lester has developed and is running a quantum Monte Carlo computer code to predict the optimal electronic pathways for photosynthetic energy transfer. A “quantum Monte Carlo” is a statistical model for studying strongly correlated systems such as electrons.
Says Lester, “Most people have long thought of computational chemistry as only being able to tackle simple systems reliably, but we’ve come a long way with improved implementation of our algorithms in recent years.”
With the INCITE grant, which will provide them with a million processor hours, Fleming and Lester are studying the electronic structures behind a defense mechanism within the photosynthetic system that protects plants from absorbing more solar energy than they can immediately utilize, and, as a result, suffering from oxidation damage. The focus will be on the carotenoids in Photosystem II, which appear to be the controlling elements behind this photoprotective mechanism.
“The photosynthetic light-harvesting system is so sensitive to changing light conditions, it will even respond to the passing of clouds overhead,” says Fleming. “It is one of nature’s supreme examples of nanoscale engineering.”
Other researchers working with Fleming and Lester on this project include Alán Aspuru-Guzik, Romelia Salomón-Ferrer, Brian Austin, Harsha Vaswani and Ricardo Oliva.
Bound for DC, Schroeder Follows Long Tradition of Service in Nation’s Capital
 Lee Schroeder, pictured in his Lab office, prepares for his extended stay
in Washington.
Lee Schroeder, pictured in his Lab office, prepares for his extended stay
in Washington.
The official acronym to describe nuclear scientist Lee Schroeder’s newest assignment is IPA: Inter-agency Personnel Agreement. But it might as well be SWS: Scientist With Suitcase.
After all, this is Schroeder’s third dispatch to Washington D.C., and in each case, he and his wife Beverly pack their bags and settle in for an extended stay in the nation’s capital. But he’s happy to do it, on behalf of his profession and his colleagues. “I see this as an enabling position — enabling the community to carry out the most important nuclear science,” he says.
“This” is the IPA that began in August in the Department of Energy’s Nuclear Physics program office under Associate Director Dennis Kovar. For at least the next year, Schroeder will work out of the Germantown (MD) office, dealing with priorities in research and associated funding, reviewing projects and agreements, and interacting with the Nuclear Science Advisory Committee (NSAC) on the implementation of the current five-year plan and the development of the next one.
“No matter what the outcome of the election, there will be budget issues,” he says. “One of the big questions facing nuclear science is, will RIA (Rare Isotope Accelerator, pronounced REE-uh) go forward?
It presently has CDO (the mission needs statement) and is poised to move forward. It’s a near-billion-dollar project and among the highest priorities of the DOE Office of Science for the construction of new facilities.”
RIA will be the world’s most powerful research facility dedicated to producing and exploring new rare isotopes that are not found naturally on earth. The beams in RIA will be 10 to 100 times more powerful than those available today. It will allow physicists to explore the structure and forces that make up the nucleus of atoms, learn how chemical elements that made up the world were created, and play a role in developing new nuclear medicines and techniques.
He also notes that nuclear science will play an important role in the global future, especially in areas like energy production. With the fossil fuel production curve going down, societies will be faced with alternative energy choices, one of which is the very controversial nuclear energy question. “In the U.S. especially, we will have to confront this question,” Schroeder predicts, “even though it’s highly politicized. We know we can produce it (nuclear energy), but can we handle the waste, and how do we as a society deal with safety? We must confront these questions in an unemotional way.
Drawing on experience
The DOE and other federal programs request interagency personnel exchanges like Schroeder’s to take advantage of the experience and the knowledge gleaned from years of successful research on the front lines. Berkeley Lab physicist Moishe Pripstein just returned from a two-year IPA assignment working as program manager for the U.S. effort at the Large Hadron Collider (LHC), due to go on line at CERN in Switzerland in mid-2007. Both the DOE and the National Science Foundation are involved, as well as close to 800 researchers and engineers across the country.
Pripstein described his experience in Germantown as “answering and sending a lot of e-mail, talking on the phone a lot, organizing reviews, and walking the halls of DOE and poking my nose in where I probably shouldn’t.” Modesty prevents him from acknowledging his success connecting the physics practitioners in the field with the agencies in Washington, a bridging role with which he is most comfortable. He says such communication is critical as program priorities are reassessed.
 Moishe
Pripstein just returned from his IPA assignment in Washington.
Moishe
Pripstein just returned from his IPA assignment in Washington.
Arriving at the Lab full-time in 1965 with a doctorate from UC Berkeley in hand, Pripstein has been involved for five decades in some of the most significant physics experiments to develop here, including the ill-fated Superconducting Super Collider and the BaBar b-factory at SLAC, for which he was head of the Berkeley Lab group. After BaBar was running smoothly for several years, Pripstein says he sought the LHC coordinating role.
“To be frank, I had been very fortunate here (at Berkeley Lab) to get well supported for my research and my ideas,” he recalls. “I felt I should give something back.” Committed for one year, he ended up staying for two.
Pripstein emphasized how significant Schroeder’s role will be. “It’s especially important now, because with the budget difficulties facing us in the future, it will be important to reassess priorities in the field. The agency, and the field, are at a critical juncture,” he says.
National Energy Research Scientific Computer Center scientist Craig Tull also departed this fall as a detailee in the high-energy physics office of DOE, coordinating computing systems planning for the LHC and other program projects.
Schroeder’s first long-distance experience was in 1987-1989, when he was detailed to DOE Nuclear Physics as a manager of the $70 million Heavy Ion Program. His expertise was coveted because of his work on the start-up team for Berkeley Lab’s heavy ion program in the 1970s, and his subsequent service as both scientific coordinator and scientific director of the old Bevalac.
Again in 1992, he was called to Washington to be assistant director for physical science and engineering in the White House Office of Science and Technology Policy (OSTP). In the administration of George Bush Sr., he had a huge portfolio that included responsibilities with DOE, NASA and the National Science Foundation, among others. With an administration change, he returned to Berkeley and, from 1995 to 2002, he directed the Nuclear Science Division.
“I’ve been a scientific bureaucrat for a long time,” he laughs, noting that can be a good thing when responding to the inevitable frustrations of process, priority-setting, and red tape. “You have to respond to it,” he says. “You can’t short-circuit it.”
Schroeder has seen it all — or most of it — in his 33 years as researcher and manager since arriving at Berkeley Lab in 1971. And DOE’s Kovar hopes to take advantage of all three decades in the coming year.
Flea Market
- AUTOS & SUPPLIES
- ‘99 FORD RANGER, 72K mi, 2.5L/4 Cyl, pwr steer/brakes (ABS), 5 sp trans, new Goodyear tires, Aiwa CD, toolbox line-X bed liner, diamond plate bed caps, tinted rear win, Scott, X4356, 220-5725
- ‘97 GEO METRO LSi, 4 dr, blue, auto, dual fr airbags, pwr steer, CD/radio, new brakes, 40 mi/gall, exc running cond, 49K mi, $2,600, Keni, X7393
- ‘95 JEEP GRAND CHEROKEE, 4 wd, 97K mi, auto, 6 cyl/4L, all pwr, ac, cruise, ABS, tinted win, good cond, orig owner, $4,800, Lauretta, 633-2052
- HOUSING
- ALBANY, junior 1 bdrm, furn, avail now, move-in date flex, 400 sq feet, dw, oven, range, microwave, DSL, nr shop/pub trans, cats ok, no dogs, $950/mo, $950 sec dep, non-smok, Jennifer or Martin, 486-6554 (w), 527-2669 (h), [email protected]
- BERKELEY, lge furn room in historic brown shingle N. Berkeley home, easy walk to Lab shuttle, downtown, BART, shops, non-smok, some kitchen privil, avail after 10/1, house has usual amenities, $500/mo, Rob, 843-5987
- EL CERRITO, fully furn spacious studio, kitchenette w/ new appliances, all util incl, free cable TV & laundry, 15 min to UCB, parking, nr El Cerrito Pl, 7613 Errol Dr, $850, 525-8211, Voya
- KENSINGTON, fully furn 3 bdrm home, view, quiet setting, 1 cat, avail for vis scientist during fall/winter terms, $1,400-1,600/mo+sec dep (depending on family size), Ruth, 526-2007, 526-6730
- KENSINGTON, furn room, own 1/4 bath, kitchen & laundry avail, $550/mo, Ruth, 526-2007, 526-6730
- KENSINGTON, fully furn in-law, all util incl, avail 10/8/04, $900/ mo+sec dep, Yoko, 528-0650
- POINT RICHMOND, 1 bdrm & 2 bdrm apts in 8-plex, Pt. Richmond Village, spacious, laundry, offstr parking, extra storage, water/hot water/garbage incl, avail now, $900/mo for 1 bdrm & $1,100 for 2 bdrm, Evan, X6784
- SAN FRANCISCO, new 1 bdrm condo at the luxurious Metropolitan (www.themetsf.com) condominium, next to Bay Bridge, walk to fin dist, 24-hr sec, indoor pool/sauna/gym/ theater & more, sophist architecture + full service living, $2,000/mo, Chai, (925)-377-6888, [email protected]
- HOUSING WANTED
- VISITING PROFESSOR & FAMILY from Finland looking for 2+ bdrm house or apt w/ good size kitchen, quiet & nice location in/around Berkeley, less than 1 mi to Lab shuttle/BART, avail 11/1/04 – 3/31/05, [email protected], [email protected], X6591
- WANTED
- RELIABLE BABYSITTER, twin girls, 2.5 yrs old, Ulli, X5347, 527-6643
- MISC ITEMS FOR SALE
- CLOTHES DRYER, Amana electric, white, 1 yr old, runs perf, $100, Steve, X6228, (925) 631-0668
- DEHUMIDIFIER, portable, 120 volt, hardly used, $40, John, 849-1051
- ROOF RACK SKI CARRIER, Barrecrafters, $20; tire chains, fits tires 8.25-14, 7.75-15, 205/75R15, 215/70R14, etc., $10; VCR, Mitsubishi HS-421UR, $25, Bill, X7735 or (925) 932-8252
- SF OPERA TICKETS, balc pair, 2nd row ctr, Sat eves, Cosi Van Tutte Oct 2, Tosca Oct 16, $124/pair, Paul, X5508, 526-3519
- FREE
- MARTHA STEWART LIVING magazines, 2 boxes +10-yr index, Kathy, X4931, can deliver to Lab
- VACATION
- LAS VEGAS, NV, time share, Polo Towers, 5-star, 2 bdrms, 1 wk every other year or 1 bdrm, 1 wk every year, floating time, highest exchange rating w/ resorts around the world, www.resortime.com, $17,000/ neg, Mustafa, 451-1350, or X5081
- PARIS, FRANCE, near Eiffel Tower, furn, eleg & sunny 2 bdrm/1 bth apt, avail yr-round by wk/mo, close to food stores, restaurants, pub trans, Geoff, 848-1830
People, AWARDS & HONORS
Sposito Wins Geophysical Prize

The American Geophysical Union (AGU), the world’s largest society of earth scientists, has selected Garrison Sposito of Berkeley Lab’s Earth Sciences Division to receive the Horton Medal “for his seminal and extensive contributions in establishing the physical and chemical foundations of hydrology.” Sposito will be awarded his medal at AGU’s annual meeting in San Francisco in December.
Cui Among Top Young Innovators

Berkeley Lab guest Yi Cui, who works in the Materials Sciences lab of Paul Alivisatos, was named to be one “100 young innovators of nanotech” by Technology Review, a publication of the Massachusetts Institute of Technology. Many of this year’s TR100 honorees, the article says, “are turning to nanotechnology to gain an unprecedented level of precision, control, and flexibility in creating new materials and devices.
In Memoriam: Ming Xie (1959-2004)

An expert in the theory of free-electron lasers and staff scientist in AFRD, Ming Xie passed away in China on Aug. 23 after a year-long battle with cancer.
Born in Beijing in 1959, Ming earned his B.S. in physics from Wuhan University in 1982. He came to the US for his graduate education and obtained his M.S. (1984) and Ph.D. (1989) degrees from Stanford University. Following his graduation, he joined Berkeley Lab as a postdoc and was promoted to staff scientist shortly thereafter. He also held the position of guest professor at Beijing University since 1998.
Ming’s lifelong interest was the theory of free-electron lasers, for which he gained wide recognition. This work led him to contribute to other areas, such as laser acceleration theory and its connection with electromagnetic radiation, gamma-gamma interactions, physics of the interaction point of future high-energy colliders, and charged-particle cooling for muon colliders. He authored or co-authored some 70 papers and contributed to the design reports on three new accelerators.
He is survived by his wife, a 16-year-old son, a sister, and his parents. His colleagues at the Center for Beam Physics are planning a memorial service