
University
Sets Fast Track for New Lab Director Search
New Hope for Malaria
Victims
University Sets Fast Track for New Lab Director Search
BY RON KOLB
|
|||||||||||
| Top
row: Blumenthal, Burnside, Clarke, Connerly, Drell Bottom row: Hopkinson, Johnson, Preuss, Seigler, Wolynes |
If the University of California has its way, the recruitment to find the next director of Berkeley Lab will be a swift one. Hoping to get the Laboratory’s leadership settled before the initial management contract competition proceeds, UC has indicated that it will try to name a successor to 15-year Director Charles Shank in May.
“We are attempting to conduct this search on a fast track so that we will be well positioned to submit a proposal for the LBNL contract,” UC Vice President for Lab-oratory Management S. Robert Foley wrote to Berkeley Lab’s senior managers.
In fact, the Joint Committee that UC chose to identify candidates
for the position met for the first time yesterday afternoon and evening
at Berkeley Lab. They spoke with groups of researchers, faculty, staff
and community representatives here in hopes of more clearly defining
the characteristics most desirable for the Laboratory’s sixth
director to have.
Shank announced his intention to step down by year’s end on
February 9, setting off a chain of actions in the University to initiate
a national search. The Joint Com-mittee, which includes five Regents
appointed by the Chairman of the Board and five others appointed by
UC President Robert Dynes, will advise Dynes during the process.
The Joint Committee is expected to receive from Dynes not less than five nor more than 15 names of candidates whom he considers promising. The Committee will then evaluate these nominations — as well as other names they may want to consider — and could interview candidates. Upon completion of the evaluation, it will advise the UC president of its findings, and Dynes will make his recommendation to the Board of Regents for consideration and approval.
The search committee includes:
- George Blumenthal, vice chair of the University-wide Academic Council and a professor of astronomy and astrophysics at UC Santa Cruz
- Beth Burnside, vice chancellor for research and professor of cell developmental biology at UC Berkeley
- John Clarke, a materials scientist at Berkeley Lab and professor of physics at UC Berkeley
- Ward Connerly, president of Connerly and Associates, Inc., and a UC Regent
- Sidney D. Drell, senior fellow at the Hoover Institution and
professor of theoretical physics (emeritus) at the Stanford Linear Accelerator Center - Judith L. Hopkinson, former chief operating officer of Ameri-quest Capital Corp. and a UC Regent
- Odessa Johnson, Dean Emerita of community education at Modesto
- Peter Preuss, president of the Preuss Foundation, Inc., and a UC Regent
- Laurence Seigler, president of the Alumni Associations of UC and an ex-officio Regent
- Peter G. Wolynes, professor of chemistry and biochemistry at UC San Diego Junior College, a member of Modesto City Schools Board of Education, and a UC Regent
The five Regents serve on the Board’s Committee on Oversight of the DOE Laboratories.
Also participating in the process are ex-officio members of the selection committee: President Dynes, who convened the group; M.R.C. Greenwood, chancellor at UC Santa Cruz and recently named provost and senior vice president of UC; John J. Moores, chairman of the Board of Regents; and UC Vice President Foley.
Discussions at the Lab yesterday were informal and confidential. The meeting included a dinner with senior Lab managers in the cafeteria. The committee was seeking opinions and suggestions about the future development of the Laboratory, plus any points that the invitees believed should be considered during recruitment deliberations.
The University’s job description for the new director notes that the appointment will be effective “on July 1, 2004, or at such a later time as is mutually agreeable to the appointee and the University.” Candidates “should have demonstrated success in leading and managing large scientific programs or organizations and should have an extensive record of scientific and technical accomplishments.”
Applications, accompanied by current resumes, and nominations may be sent to:
University of California, Office of the President, Attn: LBNL Search, 1111 Franklin Street, Room 5402, Oakland, CA 94607, or to [email protected]. They should be received no later than April 7 to be given full consideration.
New Hope for Malaria Victims
Burgeoning Field of Synthetic Biology Promises New Generation of Drugs
BY LYNN YARRIS
 |
|
| Jay Keasling, head of Berkeley Lab’s new Synthetic Biology Department, and his research team are using genetic engineering techniques to create microbes that can mass produce a powerful antimalarial drug. |
In a preview of things to come from the fledgling scientific field of synthetic biology, researchers with Berkeley Lab’s Physical Biosciences Division (PBD) and UC Berkeley’s Chemical Engineering Department are developing a simple and much less expensive means of making one of the most promising and potent of all the new anti-malarial drugs.
By adding new genes and engineering a new metabolic pathway in E. coli bacteria, the researchers can quickly and cheaply synthesize a precursor to the chemical compound artemisinin. This next generation anti-malarial drug has proven to be effective against strains of the malaria parasite that are resistant to the current frontline drugs. At present, however, it is far too expensive for countries in Africa and South America where it is needed most.
“By inserting genes from three separate organisms into E. coli, we’re creating a bacterial strain that can produce the artemisinin precursor amorphadiene,” says chemical engineering professor Jay Keasling, who is leading the research. “We are now attempting to clone the remaining genes needed for the E. coli to produce artemisinin.”
Keasling heads PBD’s new Synthetic Biology Department, the world’s first such department in a major scientific research institute. Formed by PBD Director Graham Fleming last July, the department is meant to design and construct novel organisms and biologically-inspired systems that can solve problems natural biological systems cannot, and also provide new information about living cells.
“In our research, we’re mixing different genes from different organisms in order to do new chemistry inside living cells,” Keasling says. “The goal is to enable us to produce new drugs for fighting disease or combating bioterror agents, or to produce existing drugs in better ways. That is the essence of synthetic biology and the new department we’ve created at Berkeley Lab — to harness the power of biology to solve problems that cannot be solved in any other way.”
In the medical arena, few problems have been more persistent than malaria, which was first described by Hippocrates.
According to the World Health Organization, each year nearly 500 million people living in the tropics and subtropics become infected with malaria, suffering burning fever and severe pain. Nearly three million — mostly children — die. Medical researchers have been unable to stamp out this scourge, but effective antimalarial drugs have been discovered. The best of these is artemisinin, a natural product extracted from the dry leaves of Artemisia annua, the sweet wormwood tree. Although this tree can grow in many places, it only produces artemisinin under specific agricultural and climatological conditions. China is one of the areas where artemisinin is produced, and the Chinese have been using it in the herbal medicine ginghaosu for more than 2,000 years.
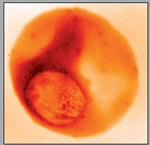
|
|
| The malaria parasite infects red blood cells, as shown by this x-ray image taken at the Advanced Light Source. | |
In recent studies, artemisinin demonstrated a nearly 100 percent success rate for the treatment of all known strains of malaria. It destroys the malaria parasite by releasing high doses of oxygen-based free radicals that attack the parasite inside iron-rich red blood cells. More than a million malaria patients have already been cured by artemisinin, but the cost of extracting the drug from sweet wormwood trees or manufacturing it through chemical synthesis is so high that the impoverished populations suffering the most cannot afford it.
Keasling and his research group have found a way to use E. coli to cheaply mass-produce artemisinin. They transplant certain yeast and sweet wormwood tree genes into the bacterium, then bypass the E. coli’s metabolic pathway and engineer a new one based on a metabolic pathway in yeast. As a result of their efforts, the yield of the artemisinin precursor amorphadiene in their laboratory strain of E. coli was increased 10,000-fold. Improvements of at least another order of magnitude are easily within reach, according to Keasling.
“The ability to produce amorphadiene in a simple organism like E. coli opens up a whole realm of possible molecular backbones that can later be functionalized to make drugs,” says Keasling. “With this ability, we can also easily encourage the bacteria to produce molecules not found in nature that could be even more effective for treating human diseases.”
Artemisinin is a terpenoid, a class of the huge isoprenoid family whose members are used in a wide variety of applications, including anticancer drugs such as taxol, as well as antimalarial drugs, plus an assortment of flavor and fragrance additives. Because terpenoids are naturally produced only in small quantities in plants, microbes and marine organisms, there’s a great interest in mass-producing them via an E. coli bacterial host. The technique used by Keasling and his colleagues should be applicable to all members of the isoprenoid family and represents a first essential step toward the production of a broad range of terpene-based compounds in microorganisms.
“We’ve taken the engineering of a microbe about as far as anyone has at this point, but it’s only the start,” Keasling says. “There are many more beneficial things that can be done with E. coli and other microbes.”
A first report on this research appeared in Nature Biotechnology last July. Coauthoring that paper with Keasling were Vincent Martin and Douglas Pitera, plus Sydnor Withers and Jack Newman. Martin holds a joint appointment with Berkeley Lab and UC Berkeley. The other three coauthors are with UC Berkeley’s Department of Chemical Engineering.
Planting the Future
 |
|
| With a nod to future generations on the Hill, the Facilities department planted eight 10-year-old redwood trees on the western slope below the 88-Inch Cyclotron. The trees, which already stand 15 feet tall, are part of an overall effort to strategically replace the many trees removed over the past years for fire safety. Bob Berninzoni of Facilities oversaw the planting, which was performed by the Facilities work crew. Last year, crews planted more than 200 oak, redwood, cherry, and crape myrtles across the Lab’s acreage. Photo by Roy Kaltschmidt, TEID |
High-Speed Connections … Circa 1960
BY D. LYN HUNTER
Long before the fax, e-mail and the Internet, Lab employees who needed to quickly transmit or receive written information stopped by the Teletype Office in Building 29.

|
|
| The "girls" of the Lab's Teletype Office included Jeannette Perry, left, and Mary Hazen. | |
Their services were profiled in an article that appeared 42 years ago in the Magnet, as the Lab’s newspaper was then called. A teletype is an electromechanical typewriter that transmits or receives messages coded in electrical signals carried by telegraph or phone wires.
“Do you have an important rush question you want to ask someone in another city? Wait before you pick up the phone — there may be a better way of getting the information,” the story starts out.
Making use of these machines, the article explains, “may not only get a speedy reply to your question, but also provide you with written confirmation of the answer for your files. And it’s usually less expensive than a phone call.“
Here’s how the Lab’s teletype service worked: The “girls,” as they were referred to in the article, connect with other organizations that have teletype machines, “and while they’re waiting for an answer they can type the message to make a perforated tape. They can then flip a switch so the taped message is relayed automatically, with no pauses. Teletype messages are also faster because they’re naturally more succinct, cutting out all pleasantries.”
Compared to today’s lightning-fast computer technology, the teletype would seem agonizingly slow. But at least the “girls” didn’t have to worry about spam, pop-up ads, or worms.
Painter Joe Cullen Keeps the Lab ‘Looking Tight’
BY D. LYN HUNTER
 |
|
| Joe Cullen |
“Tread lightly, and carry a big brush.” It is this philosophy — a tweaked version of the famed Teddy Roosevelt quote — that guides Joe Cullen’s work as a painter at the Lab. For more than 10 years, he’s helped keep the buildings on the Hill (as well as a few labs on campus) “looking tight.”
“That’s painter jargon for making things look clean and cared for,” explains Cullen. “People feel better when their work space looks nice.”
Cullen prides himself on working quickly, quietly, and neatly. “I make it a point to keep the chit-chat to a minimum, and I think the employees here really appreciate that. My goal is to be virtually invisible.”
When asked why he likes working at the Lab, Cullen says it’s the diversity: both in the many types of people that work here, as well as the different kinds of jobs he performs.
“I can be painting a bathroom in Building 7 one day, be in the Director’s office the next, then in a lab after that,” says Cullen. “And I like that the atmosphere here is casual, not too stuffy. You see people wearing all kinds of things.”
Cullen’s participation at the Lab is not restricted to just painting. This Berkeley native is the shop steward for Painters Local 3 union, a member of the Lab’s “Rated X” softball team, and, at one time, played drums and trumpet for one of the Lab’s musical groups.Working at the Lab is also somewhat of a family affair. His younger sister, Meg Holm, is an employee in the Materials Sciences Division.
When he’s not painting up on the Hill, Cullen grows vegetables at his home in El Cerrito.“I haven’t bought veggies in a supermarket in a long time,” the green-thumbed painter says proudly.
He also enjoys riding his BMW motorcycle. He uses it to commute to work whenever possible, and he also takes trips with a club. Last month they ventured to Death Valley for a three-day camping event. When he’s not riding or gardening, he’s interacting with his wife, his 14-year-old daughter and 23-year-old son.
The care Cullen applies to his vegetables, motorcycle and family he also brings to his painting at the Lab. His enthusiasm for the work shines through, despite nearly a decade of duty.
“This is the only job I’ve ever had where I truly enjoy coming to work everyday,” he says.
Berkeley Lab View
Published every two weeks by the Communications Department for the
employees and retirees of Berkeley Lab.
Reid Edwards, Public Affairs Department head
Ron Kolb, Communications Department head
EDITOR
Monica Friedlander, 495-2248,
[email protected]
Asscociate editor
Lyn Hunter, 486-4698,
[email protected]
STAFF WRITERS
Dan Krotz, 486-4019
Paul Preuss, 486-6249
Lynn Yarris, 486-5375
CONTRIBUTING WRITERS
Jon Bashor, 486-5849
Allan Chen, 486-4210
FLEA MARKET
486-5771, [email protected]
Design & Illustration
Caitlin Youngquist, 486-4020
TEID Creative Services
Berkeley Lab
Communications Department
MS 65, One Cyclotron Road, Berkeley CA 94720
(510) 486-5771
Fax: (510) 486-6641
Berkeley Lab is managed by the University of California for the U.S.
Department of Energy.
Online Version
The full text and photographs of each edition of The View, as well
as the Currents archive going back to 1994, are published online on
the Berkeley Lab website under “Publications” in the A-Z
Index. The site allows users to do searches of past articles
Better Chemistry Through Femtosecond Lasers
A faster, cheaper, and more accurate way to analyze solids
BY DAN KROTZ
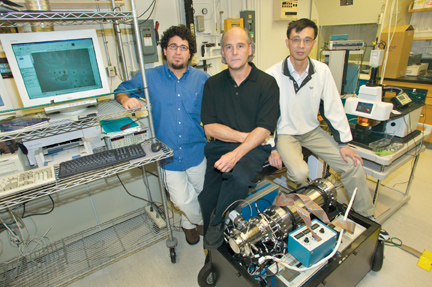
|
|
| From left to right, Jhanis Gonzalez, Rick Russo, and Xianglei Mao with a mass spectrometer used to analyze ablated particles. | |
In the quest to determine the chemical composition of solids with greater and greater accuracy, Berkeley Lab scientists are using extremely short laser bursts that span one-quadrillionth of a second.
These femtosecond-length laser bursts are used to zap a substance’s surface and dislodge an aerosolized plume of particles that can be spectroscopically analyzed. The technique is much more sensitive than similar systems that rely on longer, nanosecond-length laser bursts, and may revolutionize scientists’ ability to quickly and accurately analyze the chemical makeup of any solid — from nuclear material and hazardous waste to Martian rocks.
“It opens up the field of solid-sample chemical analysis,” says Rick Russo of the Environmental Energy Technologies Division, whose research team became one of the first in the world to use femtosecond lasers to ablate samples for spectroscopic analysis three years ago.
Funded by DOE’s Office of Nonproliferation and National Security, and the Office of Basic Energy Sciences’ Chemical Sciences Division, his team has since proven the technique’s merits. They’ve shown that femtosecond lasers are better than nanosecond lasers when it comes to analyzing the isotopic ratios of the elements that compose glass, monazite, and zircon. And they’ve reported similar results with metal alloys.
Their success lies in the enormous benefits gained when switching from laser pulses that last several nanoseconds, or billionths of a second, to several femtoseconds, which at one-millionth of a nano-second is one the fastest manmade events. Both of these laser pulses can ablate tiny regions of a solid’s surface into an aerosolized explosion, ready for analysis. But only a femtosecond laser can ablate a surface while barely heating it, thanks to the fact that nothing in nature occurs as quickly as a femtosecond, not even the movement of atoms.
In other words, a femtosecond laser pulse is there and gone before material has a chance to thermally react. This advantage sidesteps a phenomenon that has stymied laser ablation-based chemical analysis for years: elements vaporize at different rates based on their unique thermal properties. Ablate brass with a nanosecond laser, which lasts long enough to heat the compound, and zinc may vaporize while the less volatile copper remains in the sample.
“This fractionation means you’re not getting everything into the aerosol that’s in the material,” says Russo. “You’re getting things based on thermal properties, which could leave out many elements and result in a misleading analysis.”
But use a laser that’s quicker than nature can react, and every element in a substance is aerosol-ized regardless of its thermal properties. This ability to only nominally heat a surface has helped femto-second lasers make inroads into such delicate applications as the manufacture of nanomaterials, thin films, and micro-electromechanical systems. And now, thanks to Russo’s team, the lasers are poised to change the way solids are analyzed.
“At the nanosecond scale, which is the current state of laser-ablation chemical analysis, there is a tremendous amount of fractionation,” Russo says. “But a femtosecond laser explodes a sample into the vapor phase without any fractionation.”
Based on its potential, Russo believes the technique could give geologists
a better way to analyze the isotopic ratios of sedimentary layers
to determine their age. It can also analyze soil samples to map how
far a toxic plume has spread from a contamination site. To improve
national security, it can be used to test suspicious substances for
the presence of fissile material such as plutonium. And a variation
of the technique could be used in the field to search for chemical
leaks in industrial plants or to analyze Martian rocks.
Femtosecond lasers boast other advantages. In addition to barely heating
a surface, they produce an aerosol composed of a much finer particle
size distribution than a longer duration laser. A nanosecond laser
produces particles that range from five nanometers to five microns
in diameter, which is too diverse for most spectroscopic devices.
A femtosecond laser, on the other hand, yields particles that average
approximately 200 nanometers in diameter, which makes spectroscopic
analysis much more precise.
These advantages underscore how far lasers have advanced since the
early 1980s, when researchers had largely given up on their use in
chemical analysis. Back then — and until a few years ago —
longer duration laser pulses thermally damaged samples, or yielded
a hodgepodge of particle sizes unsuitable for analysis. Because of
these drawbacks, scientists have been forced to use more time-consuming
chemical analysis processes that involve sample preparation, acid-fusion
microwave dissolution, and chemical separations, among other steps.
“Now, with a femtosecond laser, we can take any solid sample,
hit it with a beam, and there’s the answer,” Russo says.
“People thought lasers weren’t going to work for chemical
analysis, but they have progressed tremendously in the past several
years.”
Other members of Russo’s research team include Jhanis Gonzalez,
Chunyi Liu, Samuel Mao, Xianglei Mao, Sy-Bor Wen, Jong Yoo, and Xianzhong
Zeng.
Doping Buckyballs One Atom at a Time
How to Tune the Electronics of Individual C60 Molecules
BY PAUL PREUSS
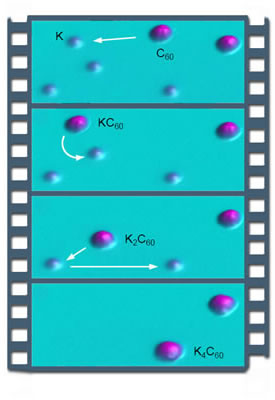 |
|
| By using the STM's tip to move a C60 molecule over potassium atoms one at a time, the buckyball can reliably be made to acquire up to four potassium-atom dopants. |
What would the 21st century be like without doping? Doping semiconductors, that is — the process of adding impurities like phosphorus or boron to materials like silicon to give the doped semiconductor an excess of negatively charged electrons or positively charged holes.
"Doping materials is a fundamental component of the entire modern electronics industry," explains Michael Crommie, a staff scientist in the Materials Sciences Division and a professor of physics at UC Berkeley. Junctions between n-type (negative) and p-type (positive) semiconductors are at the heart of the diodes, transistors, integrated circuits, computer chips, and other devices that make possible personal computers, cell phones, CD and DVD players, solar cells, and hundreds of other electronic gadgets.
Methods for doping materials in bulk are well understood, says Crommie, because "you're playing ensemble games": charge averaged over trillions of atoms is good enough. Nanotechnology is a whole different kettle of electrons. "If by nanotechnology you mean creating useful devices at the scale of 10 angstroms, then we need to take these techniques and scale them all the way down to the single-molecule level."
A nanometer is a billonth of a meter, an angstrom is 10 times smaller than that. At this level, one or two extra electrons' worth of charge can affect the performance of critical electronic components. Building a molecular-scale p-type/n-type junction might require electron doping of one molecule and hole doping of another.
Enter the STM
Recently Crommie and a team of his postdocs and graduate students used a scanning tunneling microscope (STM) to attach individual potassium atoms to isolated carbon-60 molecules, familiar soccer-ball-shaped "buckyballs." Each added potassium atom incrementally increased the negative charge on the buckyball; individual atoms could be either attached or removed using the STM's tip.
"With this work we've shown how to control the electron doping with absolute precision," Crommie says. Crystals and monolayers of buckyballs and other fullerenes have long been doped by introducing metal atoms like potassium or rubidium. Crommie and his colleagues extended the process to the atomic level by depositing widely separated C60 molecules and potassium atoms on the surface of a silver crystal polished to virtually perfect flatness. The samples were prepared in ultrahigh vacuum and cooled in the STM to just seven degrees Kelvin above absolute zero.
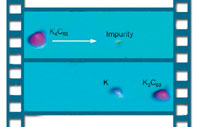 |
|
| As a buckyball acquires potassium-atom dopants the energy state of its molecular orbitals shifts, causing the doped molecule to "light up" in the STM image. | |
In an STM a voltage bias between a fine probe, only a few atoms wide at its tip, and the surface of the sample being investigated causes an electric current to tunnel between them. The strength of the current gives information about the sample's microscopic shape and electronic structure. And by bringing the tip close enough to attract individual atoms or molecules, they can be moved at will.
After depositing C60 molecules and potassium atoms on the silver surface, Crommie's group scanned the surface to map their positions. Then they used the STM's tip to maneuver the buckyballs over the potassium atoms, picking up the atoms one at a time — like a molecular Pac-Man. The buckyball's course had to be estimated by a kind of "dead reckoning," because an STM can be in either scanning mode or manipulation mode but not both at once.
Nevertheless, a buckyball could reliably be made to pick up from one to four potassium atoms. By then moving the buckyball over an impurity in the silver surface (visible in the scan but unidentified, most likely an oxygen atom), the potassium atoms could be "pulled off" one at a time.
Making the molecules dance
The shape of an individual C60 molecule did not change significantly when potassium atoms were added, but electronic changes were marked. The added charges caused the molecular orbital states of the buckyball to fill with electrons, analogous to the way the conduction band of a semiconductor fills with electrons when it is n-doped.
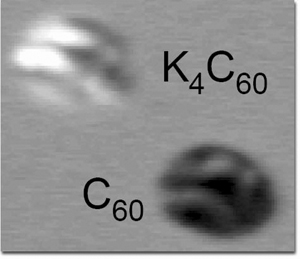 |
|
| As a buckyball acquires potassium-atom dopants the energy state of its molecular orbitals shifts, causing the doped molecule to "light up" in the STM image. |
Unlike the potassium doping of C60 in extended monolayers and bulk crystals, however, where potassium atoms contribute one electron each, here each potassium atom contributed only about 0.6 of an electron's charge to the individual buckyball. These results suggest that the potassium atoms collect at the interface where the C60 molecule meets the silver surface, partially hiding from the STM's probe and sharing part of their charge with the silver substrate.
Until now, controlling the electronic properties of molecular structures meant getting chemists to synthesize new starting materials in a test-tube, or "gating" the structure with nearby electrodes. But "if you want molecular structures to jump up and dance, the name of the game is control," says Crommie.
"Tunability is the key to tailoring the electronic properties of individual molecules," he adds. "We have demonstrated that we can do this in situ in a controllable, reversible way."
"Controlled atomic doping of a single C60 molecule," by Ryan Yamachika, Michael Grobis, Andre Wachowiak, and Michael F. Crommie, appeared in the 12 March 2004 issue of Science.
Juan Meza on ‘Amplifying’ Research, Building Diversity
BY JON BASHOR
  |
|
| Juan
Meza
Photos by Robert Couto, TEID |
Juan Meza, Berkeley Lab’s head of the High Performance Computing Research Department (HPCRD), might be the personification of the future of science. At least he would like to think so. His reputation is in two worlds — applying computing tools to the advancement of scientific inquiry, an exploding international field, and being a model for underrepresented minorities in science, diversity which he knows is essential to this nation’s future technological success. He talks about both in this InterView.
His department conducts research and development in mathematical modeling, algorithm design, software implementation, computer science and the evaluation of new and promising computer technologies. Staff members collaborate with scientists in fields ranging from materials sciences to climate modeling to astrophysics to solving computational and data management problems.
A native of Texas, Meza earned his Ph.D. and M.S. degrees in mathematical sciences from Rice University. He also holds M.S. and B.S. degrees (cum laude) in electrical engineering from Rice University.
As head of HPCRD, you’re responsible for providing the tools to help scientists increase the productivity of their research. Can you give us some examples?
Meza: I think that Anita Jones, the vice chair of the National Science Board, accurately described our role when she gave testimony to the House Committee on Science a couple of years ago. She talked about amplifying the advancement of science and engineering research through research in computer and information sciences. We often talk about accelerating scientific discovery through computation, but that’s only one part of the picture. I like the concept of amplifying scientific research. We give people the ability to do something they haven’t been able to do before, or perhaps never even dreamed they could do. That’s really at the heart of what we do.
I also like to point to the emergence of computational science and computational mathematics as true sciences in their own right. For example, a team of fusion resear-chers funded by DOE has developed a code called NIMROD to simulate fusion reactor plasmas. Through collaboration with us, this team has incorporated the SuperLU linear solver software within NIMROD. As a result, NIMROD now runs four to five times faster for cutting-edge simulations of tokamak plasmas, with a corresponding increase in scientific productivity.
Another example was described in the last issue of the View — the use of adaptive mesh refinement (AMR) in combustion. By reformulating the problem and developing new numerical methods, our researchers have developed codes that produced a 10,000-fold speedup for such simulations – enabling them to model scenarios that nobody else in the world can do. Research groups are almost fighting over who should be the next group to work with the AMR team.
Nanoscience represents a tremendous opportunity for scientific computing. Where do you see your department contributing to this effort?
Meza: Nanoscience is a perfect example of the role computational science is playing in amplifying science research. One of the major strategies behind the nanoscience modeling and simulation program was to create teams of application scientists, mathematicians and computer scientists to look at this very challenging problem.
The key issue here is scaling — most of today’s algorithms do not scale very well to large problems: The cost of the computation increases by some large power of the size of the system. For example, if you can model a system of 10 atoms in one hour and you want to increase the system to 20 atoms, the algorithm may take 50 to 100 hours to do the problem. Ideally, what we would like to have are algorithms where if you double the size of the system, you only double the time it takes to do the new computation. Then it becomes feasible to look at nanosystems where you may want to do computations with a million atoms.
Another problem is an issue we call multiscale. Although this means many things to different people, the key idea is that we need to understand systems with widely varying spatial dimensions and time scales. These are very interesting problems and I’m not sure anyone knows what the right approach is — yet.
You have co-chaired the Richard Tapia Celebration of Diversity in Computing conference, serve on the Lab’s Best Practices Diversity Council, and will cohost the upcoming Conference for African American Researchers in the Mathematical Sciences. What’s your motivation?
Meza: Diversity is a nationally important issue. If you look at the demographics for this country, the “minority” population of today will become the majority in the future. And we’re doing a poor job of helping underrepresented students prepare for careers in science and technology. In many areas, only about two percent of new Ph.D.s in this country come from underrepresented groups. I think this is a real waste of our human potential — that’s a miniscule fraction of what it should be. And the numbers for women in math and science could also be improved. If we want to keep leading in science and technology, we have to make better use of our human resources.
A second reason is one all of us deal with every day. I ask myself why we have such a hard time “selling” science and technology to the general public. One reason, I believe, is that we do such a bad job of training high school students in these areas. We are creating a population that is less and less interested in science. If we want to have a healthy state for science, we have to invest for the long term. I see my outreach efforts as doing my part toward this goal.
You’re clearly interested in helping others develop their careers in math and science. Was there someone early in your career who helped you?
Meza: At every juncture of my career there has been someone to help me understand key aspects of my profession. In graduate school, it was Richard Tapia, who helped me develop an interest in research. At Sandia, there were several people. Dona Crawford, for example, taught me many aspects of managing. In the SIAM (Society for Indus-trial and Applied Mathematics), Margaret Wright has helped me in many ways, such as understanding the importance of service to the community. And at the DOE, Paul Messina helped me to understand how to run large programs. I believe you should always look for someone to emulate or learn from.
What about your parents? Were they involved in science?
Meza: No, not really. My dad did not get a chance to graduate from high school. My mom did, and worked as a school teacher in Monterrey, Mexico. They immigrated to Texas in 1955 and I was born a year later. Both of them were very supportive and encouraging of their children. My mom certainly helped me early on. One thing she taught me was patience. She also wouldn’t let me give up. She taught me that, “If you’re going to start something, you have to finish it.” That’s something I’ve never forgotten.
OPAs: Honoring the Lab’s Best
Three times a year, Berkeley Lab’s best and brightest are honored for their contributions to the Laboratory and its programs. Put them all together, and you get a pretty good idea of what “excellence” looks like.
 |
|
|
Members of the Berkeley Structural Genomics Center team pose after
receiving their OPA from Deputy Director Sally Benson. From left are: Sung-Hou Kim, Bruno Martinez, Rosie Kim, Hisao Yokota, Benson, Paul Adams, Marlene Henriquez, Steve Xu, Barbara Gold, Dong Hae Shin, Candice Huang, Andy DeGiovanni, and Jinyu Liu. |
|
The most recent class to receive “Outstanding Performance Awards” — OPAs, as they are commonly known — includes 68 scientists and staff who were honored, as individuals or as parts of teams, for “significant one-time achievements in pursuit of the accomplishment of labwide objectives above and beyond regular job expectations.”
Managers and supervisors are invited to nominate employees, based upon what is described as “exceptional, outstanding” effort that surpasses performance goals. That could mean a significant scientific, administrative or technical contribution leading to important progress, innovation or completion of a project. Or it could have resulted in a significant improvement in cost or efficiency of an operation.
The next of the three annual OPA nomination periods ends on Monday, March 29. In the meantime, welcome to the initial members of the distinguished OPA Class of ’04.
Performance Award Winners
Jane Baynes
Coordinated negotiations with UC on management issues
Operations
Joseph Burrescia
Prepared management for successful DOE review of ESnet
ITSD
Shane Canon
Installed grid monitoring software for PDSF facility integration
NERSC
John Chernowski
Managed initiative leading to certification of Lab’s self-assessment
program
EH&S
Michael Collins
Developed historical data for use in DOE review of ESnet
ITSD
Michael DeCool
Installed and aligned successful new monochronometer at ALS Molecular
Environmental Sciences Beamline.
Engineering
Joseph Eto
Participated in international study of northeast blackout, analyzing
electric system reliability
EETD
William Fortney
Reconstructed financial history for review of ESnet by DOE
ITSD
James Gagliardi
Recreated ESnet circuit financial information for DOE review
ITSD
William Golove
Received Federal Energy Management Award for assistance in U.S. Postal
Service projects
EETD
Neil Hartman
Advanced ATLAS detector project via innovative work on coolant fitting
for high energy physics
Engineering
Gizella Kapus
Reconstructed financial records and documents for DOE ESnet review
ASD/ICSD
Stanley Kluz
Maintained stability of ESnet infrastructure during DOE review
ITSD
David Konerding
Prepared demo for Science Grid Project at Super Computing ’03.
Computational Research
Anthony Marquez
Redefined ALS gas ordering system, including safety and administrative
improvements.
Business Services
Meredith Montgomery
Led an Audit Investigations Group and delivered an important transactions
report.
Operations
Iwona Sakrejda
Installed grid middleware and applications for integrating PDSF facility
into grid infrastructure
NERSC
Tolek Tyliszczak
Reconstructed the relocated ALS beamline 7.0 scanning transmission
x-ray microscope
Chemical Sciences
Duo Wang
Developed technology to allow duct sealing in commercial buildings
EETD
June Wong
Supported two managers in successful transitions to expanded roles
ASD/EH&S
Linda Wuy
Set up viable organizational and administrative structures for interim
CFO during transition period
ASD/Financial Services
Alexander Zholents
Initiated 3-party negotiations for high-powered electron linac for
civilians, part of International Proliferation Prevention project
AFRD
Project Team:Jim Dahlgard and Jeremy Coyne
FALS User Initiatives: Proposed and implemented administrative processes
to improve User services
ASD/ALS
Project Team: Sung-Hou Kim, Rosalind Kim, Paul Adam, Barbara Gold,
Hisao Yokota, Marlene Henriquez, Bruno Martinez, Andy DeGiovanni,
Candice Huang, Yun Lou, Natalia Oganesyan, Qian Xu, Jinyu Liu, Shengfeng
Chen, Jarmilla Jancarik, & Monica Miller.
Berkeley Structural Genomics Center: Made outstanding progress toward
structural complement of two related human and animal pathogens
Physical Biosciences and ASD
Project Team: Joyce Cordell, Barbara Ahlquist, Joanne Lambert, and
Julia Turner
EETD 30th Anniversary Celebration: Coordinated successful commemorative
event, including talks, posters, and a reception
EETD and ASD
Project Team: Brent Draney, Eli Dart, Howard Walter, William Johnston,
John Hules, and John Shalf
ETF Proposal: Developed a ground-breaking proposal to the NSF for
a terascale computing facility
Computing Sciences (NERSC, ITSD, Computational Research)
Project Team: Eleanor Lee, Dennis DiBartolomeo, Christian Kohler,
Howdy Goudey, Robin Mitchell, Robert Clear, and Danny Fuller
New York Times Building: Successfully completed 3-month program to
support design of test facility for lighting efficiency at new Times
headquarters
EETD
Project Team: Howard Matis, Eric Norman, Margaret Norris, Robert Fairchild
II, Gary Zeman, and Eleanor Blakely
Radiation Presentations to Berkeley Firemen and First Responders:
Gave City of Berkeley key training in basics of radiation
Life Sciences, Nuclear Sciences, EH&S
Project Team: Karen Springsteen and Adele Syler
S&E Salary Planning Modeling: Redesigned planning worksheet for
pay increases, which will serve as model for future reviews
Human Resources
Flea Market
- AUTOS & SUPPLIES
‘02 TOYOTA HIGHLANDER, AWD, V6, 12K mi, blk, fully loaded, leather, ext warranty, maint prepaid, lojack, like new cond, $27,000, Sherry, X4115, 236-9310
‘98 FORD ESCORT SE sedan, 4 dr, 68K mi, exc cond, serv records, ac, am/fm cass, electric win & locks, cruise, pwr steer, airbags, $4,100, Nadine, X5468
‘97 TOYOTA CORROLLA, 89K mi, auto, am/fm stereo, fr airbags, ac, red sedan, clean, $4,200/bo, Hassan, X5657, 452-0421
‘95 TOYOTA CAMRY, LE sedan 4 dr, auto, 64K mi, JBC CD player w/ 12 CD changer, sliding sunrf, $7,300, 205-4883
‘95 STARCRAFT TENT TRAILER STARMASTER XL, 1224, in/outside shower, cassette toilet, 3-way ref, 2-way water heater, heater, pressurized water syst, 2 queen beds, sleeps 6, good cond, storage space, $3,500, Richard, X6616, (925) 228-2145
ALLOY WHEELS 16", 6 lug, for Toyota trucks, $200 for set of 4, Linda, (530)342-8204
MOTORCYCLES
‘02 620ie MONSTER DUCATI motorcycle, yellow, beautiful, 5K mi, fairing, $5,500 Bob, 304-3711
‘01 YAMAHA YZ125, runs great, low hrs, new renthal bars, fmf spark arrestor, $2,800, Linda, (530) 342-8204
HOUSING
NORTH BERKELEY, by wk or mo, walk 3 bl to UCB, lge fully furn 1/1 flat in Victorian dup, quiet & comfortable, laundry rm, priv garden, gated carport, nr downtown rest/BART, Geoff, 848-1830, [email protected]
NORTH OAKLAND, rm avail in 3 bdrm apt on Alcatraz nr Telegraph, BART/shops, nice apt complex w/ yard, patio, parking, mo lease, $600/mo, rent +$735 dep, Owen, X5462, 655-8498
NORTH OAKLAND, sm 1bdrm cottage, ideal for single person, laundry facil on site, non smoker pref, avail immed, $800/mo incl util, Diane, 652-7080, [email protected], Liz, X6179
OAKLAND, 3 bdrm/2.5 bth, jacuzzi in master bth, 2-car garage, 2-story townhouse, 1 bl to Lake Meritt, walk to BART, fp in liv rm, priv patio, lots of closet space, fridge, dw, w&d, avail 5/04, $1,950/mo, 2638 Harrison St, Oakland, Julia, 749-9974
SOUTH BERKELEY, 1 bdrm remod apt, unfurn, 7-unit bldg w/ character, quiet, 15 min walk to campus, nr bus/shopping, park, util incl, $1,000/mo, Kathy, 482-1777, 326-7836, Guy, X4703
HOUSING WANTED
SHORT TERM HOUSING for June & July ‘04 for 2 adults & 2 dogs (3-yr-old Australian Shepherds), seek something under $2,200/mo, dogs are well behaved & neat, one of us is a professor, Bruce, X5489
MISC ITEMS FOR SALE
2500+ AMD PC, 19" MONITOR, 5.1 dolby sound, Win XP, 80GB HD, 512MB RAM, CD-RW, DVD, ethernet, 128MB RADEON 9500 Pro, less than 1 year old, $800/bo; 25" TV w/ remote, good cond, $35, Hassan, X5657
BED, twin + mat + box spring, looks new, clean, $40; Trek mtn bike, 20" for teenagers, 18 sp, good cond, $20; Amana refrig, 19 cu ft, white, 3 yrs old, looks new & clean, $200; Whirlpool washer, 6 cycles, heavy duty, good cond $70, Chris, X6901
GAS RANGE, GE XL44, w/ self cleaning oven, dig timer clock, timed bake, thermostat, 1 low/2 med/1 high-output stovetop burners, $150, Peter, X7653
MOVING SALE, all items in exc cond, coffee table, $50; Bose 301 speakers $50 for two; stereo cabinet $20; lamps, $10 ea; end table, $10; John, X7457, 635-0652
MOVING SALE, all items in exc cond, oak din rm set: table w/ 8 chairs, server & buffet table & china closet, $500/bo, items can be sold sep, Kathy (925) 283-6266, 890-6085
OLD OFFICE FUNITURE, heavy, lge, dark wd: 3 pieces: desk, credenza & 2-drawer file cabinet, ea piece has glass inlay, credenza has pullout keyboard tray & pullout shelve for printer, 2 file drawers in desk, decent shape, $400/bo, Sheri, X6743 or 482-0105
SOLID OAK CHEST, 5 drawers; 3 drawer dresser w/ lge mirror; 7 drawer desk solid oak; pix avail, bo, Mark, X6581
FREE
FIREWOOD, John, X7457, 635-0652
VACATION
PARIS, FRANCE, nr Eiffel Tower, furn, eleg & sunny 2 bdrm/1 bth apt, avail yr-round by wk/mo, nr pub trans/shops, Denyse, 848-1830
LAKE TAHOE house, 3 bdrm.2.5 bth, fenced yard, quiet sunny loc, skiing nearby great views of water & mountains, $195/night, 2-night min, Bob, (925) 945-9345
NORTH LAKE TAHOE, house at Kings Beach, quiet cul-de-sac, great neighbrhd, 1 mi from pub beach, 10 min from Northstar, 3 bdrm/2 bth, sleeps 6, $135/night; 2 bdrm/1 bth sleeps 4, full kitchen, liv rm & din area, fp, $105/night, 2 nights min+$75 cleaning fee + $75 cleaning fee, Vlad or Linda, 849-1579
Flea Market Policy
Ads are accepted only from Berkeley Lab employees, retirees, and onsite DOE personnel. Only items of your own personal property may be offered for sale.
Submissions must include name, affiliation, extension, and home phone. Ads must be submitted in writing (e-mail: fleamarket@ lbl.gov, fax: X6641,) or mailed/delivered to Bldg. 65.
Ads run one issue only unless resubmitted, and are repeated only as space permits. The submission deadline for the Mar. 19 issue is Friday, Mar. 12.
Lab Hosts Bay Area Scientific Computing Day
BY JON BASHOR

|
|
| John Tamareis, a math student at UC Davis who presented a poster, said the event had much to recommend it, including “the variety and quality of the presentations and posters, Phil Colella's enthusiasm for the subject of my poster, a lively discussion about training computational scientists, and deep insights offered to me by Professor Kahan of UC Berkeley.” |
Eighty-five researchers in computational science and engineering turned out for the Fifth Bay Area Scientific Computing Day, held on Saturday, March 13 at Berkeley Lab. The meeting is an informal gathering to encourage the interaction and collaboration of researchers in the San Francisco Bay Area.
The annual event provides a venue for junior researchers to present their work to the local community, and for the Bay Area scientific computing and computational science communities to exchange views on today's multidisciplinary computational challenges and state-of-the-art developments. The program included technical talks, a roundtable discussion, and poster presentations.
Among those attending was Alan Laub, head of DOE’s Scientific Discovery Through Advanced Computing and former dean of engineering at UC Davis, who called the meeting “very valuable.”
“People from different fields have the opportunity to get together and learn ideas from each other, all on a local scale. It's great listening to the young people in the various fields because you realize that these are the scientists of the future.”
The attendees came from industry, research labs, and universities.
The event was organized by Tony Drummond, Parry Husbands, Sherry Li,
and Osni Marques of the Scientific Computing Group in the Computational
Research Division.









