
Lab
Says Goodbye to an Old Friend
Solar to Fuel Workshop
Eyes The Road Ahead
Lab Says Goodbye to an Old Friend
Hot food, cold champagne, and heartfelt well-wishes were in ample abundance at the farewell party held on March 24 for Deputy Lab Director Pier Oddone, who is leaving Berkeley Lab to become the next director of the Fermi National Accelerator Laboratory (Fermilab).

Lab Director Steve Chu jokes with Deputy Director Pier Oddone after
offering him a goodbye gift. Oddone is leaving Berkeley Lab to become
director of Fermilab.
The cafeteria was jam-packed with employees who had come to say goodbye to a man who has served this Laboratory for 30 years, nearly half of them as deputy director. Based on the enthusiastic applause he received both before and after his remarks, and the lengthy line of people who waited to write a personal tribute in a farewell book, Pier will leave knowing that this Laboratory community held him in the highest regard.
Berkeley Lab Director Steve Chu joked that he had “shamelessly groveled” and promised to work at Oddone’s vineyards on Saturdays if Pier would remain at the Lab. Then, noting Pier’s skills as a winemaker and chef, Chu offered to help at his vineyards anyway. On a more serious note, Chu said that while Oddone’s departure from Berkeley Lab represents a loss to our institution, his move to Fermilab constitutes a gain for science.
Physicist George Trilling, who was Pier’s predecessor as head of Physics Division, spoke of Pier’s greatness as a scientist in conceiving the asymmetric particle accelerator that became known as the B Factory, for which Oddone was awarded the 2005 W. K. H. Panofsky Prize in experimental particle physics by the American Physical Society. But Trilling also spoke of Pier’s genuine warmth and humanity, and how, even as deputy director of the Laboratory, he remained a person who could be approached without fear or intimidation.
In his own remarks, Pier told three engaging stories about his career here at Berkeley Lab. He told of how the B Factory begat the Joint Genome Institute, how his leadership of the Physics Division began with a puff of white smoke at SLAC, and how his scientific career almost came to an abrupt end before it had really started. In each of the stories there was a lesson to be learned, which Pier related to his audience.
But the lesson most likely to be carried away by those in attendance, as well as those who have had the opportunity to interact with him, is that of all the many fine scientific leaders who have served this institute in the course of its 74-year history, few have connected the way Pier did.





Clockwise from top left: Lab Director Steve Chu presents Pier Oddone
with a Tiffany clock as a goodbye gift; Horst Simon signs the farewell
book; Oddone (right) chats with well wishers Moishe Pripstein, Sherwood
Parker, Herb Steiner, and Tony Spadafora; Oddone with Bo Bodvarsson;
George Trilling speaks of Oddone's contributions to science as well
as his humanity. Photo by Roy Kaltschmidt, CSO
Solar to Fuel Workshop Eyes The Road Ahead
It’s not going to be easy, and it’s not going to happen overnight, but at least some of the solutions to one of humanity’s greatest problems may be found at Berkeley Lab. Somewhere among the Lab’s collaborative blend of biologists, chemists, materials scientists, physicists, and computer scientists could lie a way to obtain a sustainable, CO2-neutral source of energy.

Daniel Kammen of UC Berkeley advises that a diverse portfolio of clean
energy strategies should be persued.
“This is the most important scientific challenge we face today,” said Lab Director Steve Chu, speaking at a two-day meeting held at Berkeley Lab earlier this week. The meeting was convened to map the challenges that must be overcome to efficiently convert solar energy into fuel or electricity. Deputy Director Graham Fleming, an international authority on ultrafast processes, including photosynthesis, organized the meeting, which drew several dozen scientists from across the Lab and other institutions.
“If we can make solar energy an organic part of our thinking at the Lab,” Chu said, “we can come up with broad, coordinated solutions to the energy problem. Scale is crucial to our success.”
A harrowing list of statistics underscores the need to quickly wean the world from fossil fuels. Pre-industrial concentration of atmospheric CO2, a greenhouse gas, was 280 parts per million. Today, it’s 370 parts per million. Energy production and use account for 80 percent of greenhouse gas emissions. And, if that were not enough, world production of oil and gas is predicted to peak within 10 to 40 years. Although energy conservation and efficiency can buy some time, fossil fuels won’t last forever. The clock is ticking.
“Compare it to fighting cancer,” said Chu. “If we don’t cure cancer in 50 years, that will be tragic. But if we don’t solve this in 50 years, life as we know it may have to change.”
The solution promises to be as difficult to grasp as it is urgently needed. Nuclear fission may be an option, but the problems of possible proliferation of nuclear material and long-term waste storage must be solved first. Fusion is a potential solution, but there are no guarantees it will become economically feasible in the near future, if ever.
Another option is solar energy, an idea that Berkeley Lab is well equipped to pursue. Surges in computing power, the burgeoning fields of nanoscience and synthetic biology, and advances in other fields make transforming sunlight into electricity or chemical fuel — at a scale that can meet the nation’s energy demands — a tantalizing possibility for the first time.
At the meeting, strategies to harness solar energy were as wide ranging as the scientific disciplines represented. Mimicking biological systems offers one avenue. Nature has found a way to convert sunlight, CO2, water and nutrients into chemical energy via photosynthesis. Likewise, perhaps synthetic organisms can be engineered to produce methane from sunlight, CO2, water and sustainable sources of nutrients. As Chu explained, such a solution may lie at the interface of biology and materials sciences at the nanoscale.
“Synthetic biology and photosynthesis can be used as a starting point,” said Chu. “Just as we learned to fly by watching birds, we can also learn to produce energy by watching nature.”
Tom Moore, a professor in the Department of Chemistry and Biochemistry at Arizona State University, advanced a similar idea by posing a couple of questions: “Can bio-inspired constructs play a role in large-scale solar energy conversion? Is biological energy conversion sufficiently large-scale to be relevant?”
 Director Chu makes the case for a CO2-neutral source of energy.
Director Chu makes the case for a CO2-neutral source of energy.
His answer is yes, and to explain, he offered what he calls the milk-cow model. People don’t synthesize milk. They let a cow do it, which has had millions of years to fine-tune the most efficient way. Likewise, in the future, scientists could engineer organisms to express designer enzymes that can be harvested for human use. These enzymes would be renewable biocatalysts that drive reactions that make chemical fuel.
“Let nature do the majority of heavy lifting in terms of synthesis,” said Moore.
Art Nozik, a senior Research Fellow at the National Renewable Energy Laboratory, discussed the efficient conversion of solar photons to fuels and electricity via photovoltaic solar cells. His work mirrors research by Berkeley Lab scientists who have created the opportunity to design and fabricate new multijunction solar cells that will have greatly improved efficiencies.
The best solution will most likely be several solutions, advised Daniel Kammen, a professor of public policy at UC Berkeley and an expert on the management of renewable energy systems. “A portfolio of approaches is needed. Diversity is our greatest ally,” he said.
At this early stage, Kammen said that research should encompass a myriad of approaches, including low-cost photovoltaics, low-cost energy storage, biomass gasification across scales of applications, mini electric utility grids and distributed systems, and carbon sequestration. In addition, both basic research and policy analyses are needed to open new opportunities for a low carbon economy.
“Bottom line, despite some important successes, a sea change is needed. We need to get to a 70 percent reduction in our fossil fuels,” said Kammen.
Mark Levine, director of Berkeley Lab’s Environmental Energy Technologies Division, also stressed that a mix of strategies is required. “If we are to reduce greenhouse gas emissions, then we need policies, energy efficiency technology, and zero carbon supply technology. We also need to take a serious look at energy efficiency and help developing countries embrace energy efficiency technologies.”
Whatever the solution — or solutions — the closing question in Chu’s talk both framed the problem and posed the challenge: “Who will keep the lights on after we run out of fossil fuel?”
In the Breakouts
During Tuesday’s wrap-up session of the Solar to Fuel workshop, Lab Director Steve Chu remarked that the workshop had succeeded in “identifying the hard problems.” One of the hardest was characterized by Paul Alivisatos, director of the Materials Sciences Division (MSD), as maintaining the right balance between the possible and the practical.

Paul Alivasatos
“It’s not good for scientists to constrain their imaginations,” Alivisatos said, “but as our people pursue their ideas we must keep them informed of all the practical realities out there.”
Alivisatos was enlarging on a theme he had introduced at one of the previous afternoon’s four breakout sessions, the one on “Nanomaterials and Nanostructured Assemblies for Photochemical Conversion,” chaired by himself and MSD’s Peidong Yang. There are two broad approaches, he said, to the fundamental questions posed by the challenge of designing nanostructures to turn light into electricity or chemical fuel.
“One way is to seek perfection. We already have multilayer solar cells that can convert sunlight to electricity with 37 percent efficiency — in the laboratory. So one might suppose it’s only an issue of scaling up to mass produce these devices cheaply.”
But that’s not likely to happen. An alternative could be low efficiency but very cheap materials like plastic. Alas, organic materials have an unfortunate tendency to break down during exposure to sunlight; once the chemical pathways for a material to reconstitute itself are built in, any price break might vanish — an example of the real world raising caution flags for a novel idea.
The sessions identified hard problems aplenty. Steve Chu’s advice to researchers: “let these problems run in the background of your day-to-day thinking, so that you are subconsciously thinking about several big and important problems.”
Already ideas are fermenting. Leaving the workshop, people were musing about borrowing ideas from biology without trying to mimic biological systems; it’s a good bet that ideas for modifying living plants, borrowed from the chemical and physical sciences, are on the back of the envelope as well.
Mars Still a Suspect for Origins of Life
Some four billion years ago, Earth in its infancy, was in the throes of cataclysmic bombardments from space, engulfed by superheated steam under a rock-burning atmosphere of 3,000 degrees Celsius. Meanwhile, our planetary neighbor — now a freeze-dried, meteorite battered desert — was enjoying Mars Spring. During its brief, one-million-year-old active life, the Red Planet had water running in its now-dry riverbeds and was bubbling with volcanic activity. This combination created hospitable conditions for life to form. Then, when struck by comets, large rocks blasted off Mars and traveled through the solar system, taking along for the ride tiny colonists — microorganisms that would eventually find their way to Earth and give rise to life on our now-blue and biofriendly planet. We are its descendants.

Astrobiologist Paul Davies shows an illustration what the Earth must have looked like during its first billion years of turmoil. Under those conditions, he said, life was unlikely to form or survive on our planet.
Science fiction? Not necessarily, according to proponents of Martian biogenesis such as Paul Davies, who made his case at Berkeley Lab in a guest lecture on March 24. A professor of natural philosophy at the Australian Centre for Astrobiology at Macquarie University, Davies is a theoretical physicist, astrobiologist, and best-selling author of 27 books, including The Fifth Miracle: The Search for the Origin and Meaning of Life.
This theory of a “second genesis” turns on its head conventional wisdom of how life evolved — what he refers to as the “party line for bio-origin for over a century” — the notion that life sprung out of the proverbial primordial soup on Earth.
Davies bases his case on a timeline which, he says, gives evolution precious little time to produce the fossil record on Earth. Rocks 3.5 billion years old found in Western Australia, he explained, bear traces of life that scientists believe include fossilized microbes.
“This raises an issue,” Davies said. “Life wouldn’t have sprung into existence ready-made. There would have been a long period of evolution leading up to this.”
But when? During Earth’s first million years, life couldn’t have formed or survived here, he said. The collisions with space objects were so violent, the Moon was stripped off, and its craters still bear witness to the impacts of the time. The bombardments did not abate for another 700,000 million years, which would bring us suspiciously close to the age of those Australian rocks.
“There are two ways of avoiding this conflict,” Davies suggested. “Put life underground, or put it on Mars.”
Addressing the first alternative, Davies debunked the long-held premise that sunlight and photosynthesis are critical to sustaining life. Bioorganisms have been found deep beneath the sea bed in total darkness. The only problem with placing our ancestors there is that the tiny organisms had no obvious reason to evolve, since the conditions there have not changed much.
Enter the sexier alternative. Instead of rising from below, life reached us from the heavens above. Today, Mars is dead tectonically and has only one percent of Earth’ atmosphere. But in its glory days, life could have sprung and survived on the Red Planet long enough to be propelled to other worlds. Microorgan-isms flying through space “could survive and come out smiling,” Davies said, killed neither by impact, heat, nor radiation during their interplanetary journey.
Davies acknowledged that there is no evidence to date of life beyond Earth, let alone for his scenario. Nor is Mars the sole suspect, should we look beyond our planet for the seeds of life. Indeed, life could have formed in yet another place and then colonized one or both planets.
Whichever world spawned the miracle of life, Davies said he hopes we’ll eventually find evidence of its existence on Mars. The next step then would be to determine if the life forms are similar — in which case one of the planets colonized the other. Or, even more interestingly, he said, if different forms of life were to be found, this would prove that the universe has biofriendly laws.
Davies’ talk ended with philosophical musings about whether life happened by chance or law, about biology and determinism, and even about the very definition of life.
While the theory of Mars biogenesis remains in the speculative domain, scientists and nonscientists alike remain fascinated by the mystery of the Red Planet, and many can’t help wonder … what if?
“Could we be all alone,” Davies asked, “or is this wishful thinking?”
The Goldhabers Collaborate on Aesop Fables\ Sonnets From Aesop
by Judith Goldhaber, with illustrations by Gerson Goldhaber Ribbonweed Press; 209 pages; $14.95
“Sour grapes,” “never cry wolf,” “dog in the manger” — a great many perennially apposite phrases entered our language from the fables of Aesop, who was born a slave in the late sixth century BC but, if Herodotus is to be believed, was freed by his admiring master and earned fame throughout the Greek world. His timing was right. Outspoken democracy was on the rise; Aesop’s tales — many of them (but not all) featuring talking animals and most (but not all) mocking human foibles — gave voice to the opinions of common citizens, unlike the high-falutin’ odes of aristocrats like Pindar.

Aesop’s fables have been reinvented many times since. More than 500 are attributed to him (many of them repetitious, however). But the present selection and retelling of 100 fables by Judith Goldhaber, formerly a member of Berkeley Lab’s Communications Department, is among the cleverest and most successful collections yet. Each of her bright, witty sonnets is perfectly reinforced by an illustration painted by her husband, Gerson, of the Lab’s Physics Department, in what they describe as their first collaboration “not counting their children and grandchildren.”
As a vehicle for telling fables, Goldhaber’s choice of the sonnet was inspired. Fourteen lines is just the right length for a narrative with a point, and the sonnet’s rhyming conventions give rise to many oppor-tunities for word-play that she exploits with obvious delight. “The Hare and the Tortoise” begins this way:
“Hey, Stumpy! Is that you or just some rock?”
a rude Hare ridiculed his friend the Tortoise.
“Are you alive, or is that rigor
mortis?”
Goldhaber doesn’t shackle herself to the canonical forms of the sonnet (indeed, the last poem in the volume, “The Man, the Boy, and the Donkey,” takes 18 lines to get to its point, “please all, please none”), but instead ends each in a rhyming couplet, reinforcing surprise with anticipation. From “The Mule”:
“... I thought myself a horse, but now, alas,
I’m pretty sure my father was
an ass.”
Gerson Goldhaber’s watercolors and mixed media illustrations are the visual counterparts of these lively poems, humorous, balanced compositions in vivid colors full of energy — true storytelling pictures reminiscent of the work of Marc Chagall.
In making these fables pertinent, the Goldhabers have not hesitated to introduce phrases and places Aesop never heard of (“The Boy Who Cried Wolf” is set in Afghanistan), but unlike adaptations that assume homilies were all he had in mind, these stay true to Aesopian roots. Not all the sonnets tell funny stories with morals; some give a glimpse of the ancient world from which they came, as in Aesop’s version of how the olive tree became associated with Minerva (Athena).
And some are simply touching, as in the tale of “The Lark Burying Her Father.” A lark has traveled the universe seeking a fit place to bury her father:
To honor her devotion, Zeus made the Earth:
So one Lark’s death became our green globe’s birth.
To order copies of Sonnets From Aesop, visit http://www.sonnetsfromaesop.com/, or call Gerson Goldhaber at X6210.
Lights, Camera, Action
Lab Engineer Directs Award-Winning Short
 Lab mechanical engineer Neal Hartman nurtures his creative side by
directing films.
Lab mechanical engineer Neal Hartman nurtures his creative side by
directing films.
Growing up, Neal Hartman was split between his love of science and artistic proclivity. He attended an arts magnet junior high school, where he focused on writing and producing television and film. But after that, he went to a high school that specialized in science and math.
“By the time college rolled around, I had to make a choice,” he recalls. “So I decided to major in mechanical engineering at UC Berkeley.”
Hartman started working at the Lab while a student, then as an intern, and now as an employee of the Engineering Division matrixed to the Physics Division.
“I put my artistic stuff on the back burner while I pursued my degree and career,” says Hartman. “But a few years back, I decided to reconnect with my creative side.”
After taking some film classes from a community college, he was ready to ply his craft. And the opportunity arose when he heard about a unique competition in San Francisco called “The 48-Hour Film Project.”
“You basically get your assignment on a Friday evening, then have to turn in a finished product by Sunday night,” Hartman explains. “When I first tried in 2003, it was a total disaster. But this past year I was a lot more prepared.”
The competition requires filmmakers to pick a genre, a character, a line of dialogue, and a prop — each written on a piece of paper — out of a hat. All four must be used in the film.
 In
a scene from Hartman’s film, punk rocker D. Nightengale beats
up Jackson, an over-zealous meter maid.
In
a scene from Hartman’s film, punk rocker D. Nightengale beats
up Jackson, an over-zealous meter maid.
“We got comedy, a character named D. Nightengale, the line ‘You’re in the groove,’ and the prop was a dog,” Hartman says. “We took this information and immediately started creating ‘The Ticket.’”
Hartman and his team, which included Engineering Division colleague Alexis Smith and Aldo Saavedra (now with the Large Hadron Collider in Switzerland), came up with a clever story about an over-zealous meter maid who gets his come-uppance after an altercation with a punk rock diva.
“It’s a story about personal revelation through hardship,” says Hartman with a sly grin. “And we told it in about six minutes.”
The film won first prize (out of 22 submissions), as well as the audience award, best writing, and best use of assigned dialogue (the Jackson character gets his foot stuck in a crack in the sidewalk, which prompts his sidekick to say “You’re in the groove.”).
“It’s amazing what you can do in just two days with no sleep,” says Hartman. “You surprise yourself all the time, and in the end, the results are more than you would ever expect.”
Follow the Energy: New Technique Enables Scientists to Track Molecular Energy Transfer in Photosynthesis
In the “Solar to Fuel: Future Challenges and Solutions” workshop held at Berkeley Lab this week (see front page story), photosynthesis was a major topic of discussion. Through the photosynthetic process, green plants and cyanobacteria are able to transfer energy from sunlight and initiate its conversion into chemical energy with an efficiency of nearly 100 percent. If we can learn to emulate nature, we, too, could effectively tap into the sun as a clean, efficient, sustainable and carbon-neutral source of energy for our technology. First, however, we need a much better understanding of how photosynthesis works at the molecular and electronic levels.
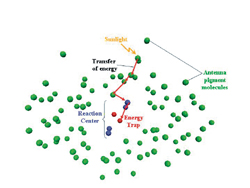 Photosynthesis starts with a photon of sunlight being captured by
an antenna pigment molecule, such as chlorophyll. This generates energy
(red arrow) that is transferred through a series of molecules until
it is trapped in a reaction center where the solar energy is converted
to chemical energy. (Image by Harsha Vaswani)
Photosynthesis starts with a photon of sunlight being captured by
an antenna pigment molecule, such as chlorophyll. This generates energy
(red arrow) that is transferred through a series of molecules until
it is trapped in a reaction center where the solar energy is converted
to chemical energy. (Image by Harsha Vaswani)
An important step towards learning more about how nature efficiently transfers energy from one molecule to another was reported in the March 31 issue of the journal Nature. A team of Berkeley Lab and UC Berkeley researchers, led by Lab Deputy Director Graham Fleming, was able to follow the flow of excitation energy in both time and space in a molecular complex using a new technique called two-dimensional electronic spectroscopy. In doing so, they made a surprise finding.
“I think our technique will prove to be a revolutionary method for studying energy flow in complex systems where multiple molecules interact strongly,” said Fleming, an internationally-acclaimed leader in spectroscopic studies of the photosynthetic process. “Using two-dimensional electronic spectroscopy, we can map the flow of excitation energy through space with nanometer spatial resolution and femtosecond temporal resolution.”
Fleming, also a professor of chemistry with UC Berkeley, was the principal investigator and co-author of the Nature paper, entitled “Two-Dimensional Spectroscopy of Electronic Couplings in Photosyn-thesis.” Co-authoring the paper with Fleming were Tobias Brixner, Jens Stenger, Harsha Vaswani, Minhaeng Cho and Robert Blankenship.
Two-dimensional electronic spectroscopy involves sequentially flashing a sample with light from three laser beams, delivered in pulses only 50 femtoseconds (50 millionths of a billionth of a second) in length, while a fourth beam is used as a local oscillator to amplify and phase-match the resulting spectroscopic signals. Fleming likens the technique to that of the early super-heterodyne radios, in which an incoming high frequency radio signal was converted by an oscillator to a lower frequency for more controllable amplification and better reception. In the case of 2-D electronic spectroscopy, scientists can track the transfer of energy between molecules that are coupled through their electronic and vibrational states in any photoactive system, macromolecular assembly, or nanostructure.
 Graham
Fleming, deputy director of Berkeley Lab, led the development of a
new technique called two-dimensional electronic spectroscopy, which
enables scientists to map the flow of excitation energy through space
with nanometer spatial resolution and femtosecond temporal resolution.
Graham
Fleming, deputy director of Berkeley Lab, led the development of a
new technique called two-dimensional electronic spectroscopy, which
enables scientists to map the flow of excitation energy through space
with nanometer spatial resolution and femtosecond temporal resolution.
“This technique should also be useful in studies aimed at improving the efficiency of molecular solar cells,” Fleming said. In the Nature paper, he and his colleagues describe how they successfully used 2-D electronic spectroscopy to record the first direct measurement of electronic couplings in the Fenna-Matthews-Olson (FMO) photosynthetic light-harvesting protein, a molecular complex in green sulphur bacteria that absorbs photons and directs the excitation energy to a reaction center, where it can be converted to chemical energy.
“FMO is a model system for studying energy transfer in the photosynthetic process because it is relatively simple (consisting of only seven pigment molecules) and its chemistry has been well characterized,” Fleming said. “As in all photosynthetic systems, the conversion of light into chemical energy is driven by electronic couplings between molecules, and we monitored the process as a function of time and frequency.”
Fleming and his colleagues expected to find that the excitation energy from harvested photons in the light-capturing pigment molecules was transported to the FMO reaction center molecules step-by-step down the energy ladder. Instead, they discovered distinct energy pathways, based on the spatial arrangements of the molecules, whereby some of the intermediate steps in the energy ladder are skipped.
“Excitation energy moved through the FMO complex in a smaller number of steps but larger energy increments than was previously supposed,” said Fleming. “What we’re seeing is that nature exploits quantum mechanical effects by delocalizing excitation energy over two or more molecules in a system.”
The next step for Fleming and his researh group will be to apply their 2-D electronic spectroscopy technique to the study of the molecular systems in a photosynthetic reaction center.
“It’s not enough to just be able to harvest light efficiently; you also have to be able to efficiently convert it to a useful form of energy,” Fleming said.
New Map of the Protein Universe
A comprehensive new 3-D map that brings order to the universe of proteins has been unveiled by a team of Berkeley Lab and UC Berkeley researchers. This map provides scientists with a much-needed guide to exploration and discovery in a universe that contains an estimated 50 billion different proteins, and could actually hold trillions more.
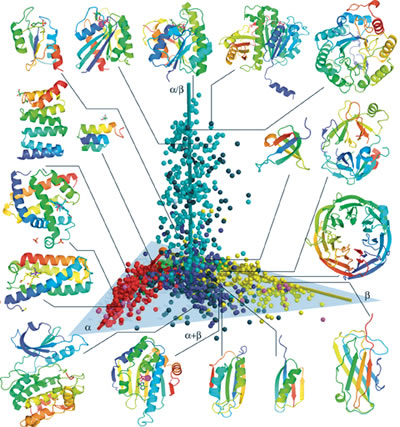 In this 3-D protein structure space map (SSM), each of the known 1,898
unique protein structures is represented by a sphere, color-coded
according to the widely used Structural Classification of Proteins
(SCOP) system. The spheres are distributed along three elongated regions.
Spheres that are close together will usually share similar structure
and function, as well as evolutionary history, making this map an
excellent predictive tool for exploring the protein universe.
In this 3-D protein structure space map (SSM), each of the known 1,898
unique protein structures is represented by a sphere, color-coded
according to the widely used Structural Classification of Proteins
(SCOP) system. The spheres are distributed along three elongated regions.
Spheres that are close together will usually share similar structure
and function, as well as evolutionary history, making this map an
excellent predictive tool for exploring the protein universe.
“We have constructed a protein structure space map (SSM) based on the distribution in (3-D) space of the 1,898 known unique protein structures,” said Sung-Hou Kim, a chemist who holds a joint appointment with Berkeley Lab’s Physical Biosciences Division and UC Berkeley’s Chem-istry Department. “Because proteins with similar structures and functions are clustered together in the SSM, when the structure of a new protein is first identified it can be placed in the appropriate location on the map to reveal its neighbors and its evolutionary history. This information can then be used to predict the protein’s function.”
Kim is an internationally recognized authority on protein structures and a pioneer in the field of structural genomics. Two years ago he led the development of the first 3-D map of the protein universe, which was based on the spatial distribution of the 498 most common protein folds — the recurring structural motifs, or “domains,” that underlie all protein architecture.
“The earlier mapping of protein fold space only looked at architectural domains, whereas the SSM looks at protein structures that may contain more than one domain,” Kim said. “We believe that this SSM is the best available method at the present for predicting the functions of new proteins whose functions cannot be predicted from their amino acid sequence information or structural similarity, the two most commonly used methods.”
As the DNA base pairs that make up the genomes of a growing number of different organisms get sequenced, the next horizon for the biosciences is to identify coding genes and the molecular and cellular functions of the proteins encoded by them. Coding genes are DNA sequences that translate into sequences of amino acids which fold into proteins. The prevailing scientific method for predicting the function of a newly discovered protein has been to compare the sequence of its amino acids to the amino acid sequences of proteins whose functions have already been identified. A major problem with relying exclusively on this approach is that while two proteins organisms may have similar structure and function, the sequences of their amino acids may be dramatically different.
“From the data available at this time, it would seem that protein structure has been much more conserved during evolution than genetically based amino acid sequences,” Kim said. “Because the functionally important portions of proteins fold into specific structures to perform their tasks, structure-based functional inference can be used to characterize close relationships between different proteins that would be impossible to detect by using amino acid sequences alone, or by the similarity of whole structures.”
Through the eons, proteins have selectively evolved into the architectural structures best suited to do their specific jobs. These structures essentially stay the same for proteins from all three kingdoms of life — bacteria, archaea and eukarya — even though the DNA sequences coding for a specific type of protein can wildly vary from the genome of one organism to another, and sometimes even within the same organism. Evidence of this conservation can be seen in the fact that, while the protein universe may encompass trillions of different kinds of proteins, most structural biologists believe there are probably no more than 10,000 distinctly different types of architectural structure motifs.
 Chemist
Sung-Hou Kim is an internationally recognized authority on protein
structures and a pioneer in the field of structural genomics.
Chemist
Sung-Hou Kim is an internationally recognized authority on protein
structures and a pioneer in the field of structural genomics.
The SSM developed by Kim and his colleagues, Jingtong Hou, Se-Ran Jun and Chao Zhang was reported in the January 5 issue of the Proce-edings of the National Academy of Sciences. In the SSM, each of the 1,898 individual protein structures is represented by a sphere, which is assigned a color according to the widely used Structural Classification of Proteins (SCOP) system. The spheres are distributed along three elongated regions, which are centered around three axes, denoted alpha, beta, and alpha slash beta.
In comparing the ability of the SSM to predict protein function to that of the most widely used current predictive method, the DALI Z scores, Kim and his colleagues found that the SSM method can often be used to predict the molecular function of a new protein whose function could not be predicted using the DALI Z scores.
“The SSM was able to reveal similar functions even between proteins that had dissimilar structures and sequences,” Kim said. “This observation suggests a scheme to infer protein functions based on the distances in the SSM, especially for proteins with new folds.”
The SSM should make it possible for researchers to quickly classify newly discovered proteins and provide a running start in identifying the functions of these proteins. It should also be a big help in developing rational strategies for the discovery or design of new drugs.
Development of the protein universe SSM was funded by grants from the National Science Found-ation and the National Institutes of Health.
Making the Buckyballs Ring
 The
deep red colors in some stained glass windows created during the Middle
Ages were the result of surface plasmons, an electronic state of gold
nanoparticles in the glass.
The
deep red colors in some stained glass windows created during the Middle
Ages were the result of surface plasmons, an electronic state of gold
nanoparticles in the glass.
Ronald Phaneuf is fascinated by what happens when energetic photons collide with ions. Such interactions are ubiquitous in the plasmas that make up more than 99.99 percent of the visible matter in the universe, where unbound electrons, positively charged atomic and molecular ions, and photons (particles of light) coexist.
“Yet there is very little experimental information on the electronic structure of ions that interact with photons in this state,” Phaneuf says, “mainly because it has been so technically difficult to do the experiments.”
So Phaneuf, who is Foundation Professor of Physics at the Univer-sity of Nevada, Reno, together with his research group and collaborators from Germany, worked with the Advanced Light Source to build a specialized research facility at ALS beamline 10.0.1, which is under the direction of David Kilcoyne of the ALS Scientific Support Group. The experimental apparatus sends a beam of singly or multiply charged ions to interact with an extraordinarily bright beam of photons produced by the beamline’s associated undulator magnet, whose energy can be tuned from ultraviolet to soft x-rays with very high spectral resolution.
In experiments performed at the ALS and elsewhere, Phaneuf and colleagues from the U.S., Germany, Brazil, Great Britain, Denmark, and Hungary have conducted numerous electronic-structure studies of photoexcited ions, including carbon, nitrogen, oxygen, fluorine, neon, scandium, titanium, manganese, nickel, and other atomic and molecular species.
By tackling the electronic structure of photoexcited carbon-60 (so-called buckminsterfullerenes, or buckyballs), however, their latest result breaks new ground. David Kilcoyne says of the experiment, “The beauty is that this beamline and this end station, plus the international team Ron Phaneuf assembled, put us far ahead in the effort to gather detailed information about such phenomena.”
Buckyballs on the cusp
Fred Schlachter, another of Phaneuf’s collaborators on the ALS staff, explains what makes a C-60 ion special: “For a molecule made from a single element, C-60 is a very large. It marks the transition from atoms to solids.”
In atoms and small molecules, the behavior of electrons is accounted individually; in bulk materials, a sea of innumerable electrons behaves en masse, yielding a very different description of electronic structure. Buckyballs perch on the cusp between these states.
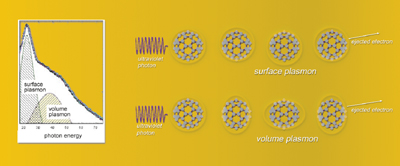 When stimulated by photons at an energy of about 20 electron volts,
a buckyball displays collective electron motion as a surface plasmon.
But when stimulated by 38-eV photons, the result is a different mode
of collective electron motion, a volume plasmon.
When stimulated by photons at an energy of about 20 electron volts,
a buckyball displays collective electron motion as a surface plasmon.
But when stimulated by 38-eV photons, the result is a different mode
of collective electron motion, a volume plasmon.
Like other nanoparticles occupying this intermediary condition, a buckyball’s electrons are capable of acting in specific concerted states, as evidenced by the discovery in the early 1990s that, when subject to excitation energy of about 22 electron volts (22 eV), the four valence electrons belonging to each of the buckyball’s 60 carbon atoms, 240 in all, act collectively, resulting in a “giant resonance.”
The collective motion first identified in C-60 was a so-called surface plasmon, a back-and-forth oscillation of the whole cloud of valence electrons relative to the effectively rigid cage of carbon cores. (Carbon atoms occupy the vertices of the hexagons and pentagons that trace out a buckyball’s characteristic soccer-ball geometry.)
Surface plasmons can produce obvious effects. A Feb. 22, 2005 article by Kenneth Chang in the New York Times notes that the deep red color in medieval stained-glass windows is actually produced by nanoparticles of gold: “Electrons at the surface of the nanoparticles slosh back and forth in unison, absorbing blue and yellow light. But longer-wavelength red light reflects off the particles.” The Times calls the stained-glass artisans “the first nanotechnologists.”
At beamline 10.0.1 Phaneuf’s group sent a fine beam of buckyball ions of the desired charge state — +1 for most measurements, corresponding to a total of 239 valence electrons — to collide head-on with a similarly fine beam of ultraviolet photons. The energy of the photon beam was tuned through a range of values, from less than 20 eV to more than 70 eV. Photoexcited ions were deflected to a detector, which measured the number of ions at different photon energies and their ion charge states.
A mysterious bump in the curve
The group’s first experiment with C-60 resulted in a clearer-than-ever picture of the giant resonance at 22 eV, evident as a sharp peak in a graph showing — as a function of the energy of the photon beam — the number of buckyballs that had lost an extra electron as a result of colliding with a photon of that energy. But instead of falling off smoothly from this peak as photon energy was increased, there was a secondary rise or shoulder in the curve.
Shortly after this experiment, Ron Phaneuf was in Berlin, where he chanced to meet a group of theorists who, solely on the basis of calculations, had predicted a higher-energy resonance than C-60’s known 22-eV giant resonance. But because of a lack of experimental evidence they had not published their prediction.
“We had an experiment looking for an explanation and they had
an explanation looking for an experiment,” says Phaneuf. “It
was a scientific marriage made in heaven.”
The second resonance in C-60, occurring at a photon energy of 38 eV, is called a volume plasmon — not a back-and-forth oscillation of the valence electron cloud but rather an in-and-out contortion, like squeezing a beach ball. Such collective motion would be impossible if C-60 were a solid sphere instead of a hollow charged shell; for this reason it has not been observed in metal clusters like gold nanoparticles, where surface plasmons are common.
A cloud of electrons collectively wobbling in and out, penetrating the cage, is a phenomenon unique to charged buckminsterfullerenes. Like hitting a big bronze bell with a clapper, it’s a way to make the buckyballs ring.
Berkeley Lab View
Published twice a month by the Communications Department for the employees and retirees of Berkeley Lab.
Reid Edwards, Public Affairs Department head
Ron Kolb, Communications Department head
EDITOR
Monica Friedlander, 495-2248, [email protected]
STAFF WRITERS
Lyn Hunter, 486-4698
Dan Krotz, 486-4019
Paul Preuss, 486-6249
Lynn Yarris, 486-5375
CONTRIBUTING WRITERS
Jon Bashor, 486-5849
Allan Chen, 486-4210
David Gilbert, 925-296-5643
FLEA MARKET
486-5771, [email protected]
Design
Caitlin Youngquist, 486-4020
Creative Services Office
Communications Department
MS 65, One Cyclotron Road, Berkeley CA 94720
(510) 486-5771
Fax: (510) 486-6641
Berkeley Lab is managed by the University of California for the U.S. Department of Energy.
Online Version
The full text and photographs of each edition of The View, as well as the Currents archive going back to 1994, are published online on the Berkeley Lab website under “Publications” in the A-Z Index. The site allows users to do searches of past articles.
Flea Market
- HOUSING
- BERKELEY, 1 bl from UCB, lge, nicely furn 1 bdrm apts w/ computer & DSL, $1,575/mo incl all util, Jin, 845-5959, [email protected]
- BERKELEY, resid community of scientists/staff/grad students, Hearst Commons, 1146-1160 Hearst nr UC/ pub trans/BART, res parking, internet, studio townhouses w/ decks, furn can be provided at no cost, hardwd flrs, skylights, dw, ac, intercom, sec, units from $850, 848-5371, http://www.live-work.us/cgi-bin/artbooks/berkeley.html
- BERKELEY HILLS, bay view, lge sunny furn rm, 17’x15’, priv ent, own bthrm, quiet neighborhood near UC/pub trans/shops, cooking facil in adj rm, pool table, workout mach, w&d, $875/mo incl linens, dishes, utils, phone line, wireless DSL, dish satellite TV/VCR, no smok/pets, short stays, $325/wk, Carol, 524-6692
- CENTRAL BERKELEY, nice furn rms, $350/wk or $850/mo, kitchen, laundry, TV, DSL incl, walk to Lab shuttle, shops, Jin 845-5959, [email protected]
- EL CERRITO, 6825A Stockton Ave,
- 2 bdrms/1 bth, nr BART & shopping, nice neighborhood, $895/mo, Daili Wang, 236-4684, cell 910-0589
- EL CERRITO, fully furn 3 bdrm/2 bth house, avail July & Aug, 20 min from Lab by car, pub trans, $1,500/mo+ dep, incl util less long dist calls, 237-4654, [email protected]
- WALNUT CREEK, 1 bdrm upper unit, nr Pleasant Hill BART, new kitchen/ appliances, 2 new air conditioners, new drapes, 733 sq ft, carport, pool, picnic area, laundry rm, exercise rm, $1,000/mo, lease, no pets, Patricia, (925)-932-3680
- MISC ITEMS FOR SALE
- BOOKCASE, teak drop-lid, 30”W x 16”D x 75”H, $35, beige lacquer dresser, 62”L x29”H x 17”D, $50, Sue, 595-7123
- COMPUTER, Toshiba laptop, 800 Mhz cpu, 14 Gb HD, TV output, ether card, USB, modem, Windows, Office, misc utilities, Steve, X4982, [email protected]
- LOVE SEAT, green w/ pink fabric, exc cond, $95/bo; twin-mattress, box & bed frame, good cond, incl bed skirt & fitted sheet, $95; floor lamp, $15/bo; a few sets of silver Shilag door knobs, misc items, Minmin, 847-5130
- MICROWAVE, Whirlpool, white, 1.2 cu-ft, exc cond, $25; Panasonic compact stereo syst w/ 3-disc CD changer, dual cass, tuner & speakers, blk, exc cond, $45; Ron, X4410, 276-8079
- SOFA, 2 six-ft sofas, frames exc, fabric torn, bo over $100 ea, Mark, X6581
- SOFA, L shaped leather sectional, 7’ per leg cream colored, good cond, no scratches, $250; wide screen, rear projection, RCA television, 46” diag, perf operating cond, $250, John, X6573
- WANTED
- HOUSESITTER, 4/21-5/2 or part, no pay but a very nice house in Oakland nr Hwy 13, care for 2 cats, Ken, X7739, [email protected]
- VACATION
- TAHOE KEYS, house, 3 bdrm/2.5 bth, fenced yard, quiet sunny location, skiing nearby, great views of water & mountains, $195/night (2 night min), Bob. (925) 376-2211
Flea Market Policy
Ads are accepted only from Berkeley Lab employees, retirees, and onsite DOE personnel. Only items of your own personal property may be offered for sale.
Submissions must include name, affiliation, extension, and home phone. Ads must be submitted in writing (e-mail: [email protected], fax: X6641), or mailed/delivered to Bldg. 65.
Ads run one issue only unless resubmitted, and are repeated only as space permits. The submission deadline for the April 15 issue is Thursday, April 7.